Nasal Delivery of Hesperidin/Chitosan Nanoparticles Suppresses Cytokine Storm Syndrome in a Mouse Model of Acute Lung Injury
- PMID: 33584267
- PMCID: PMC7873598
- DOI: 10.3389/fphar.2020.592238
Nasal Delivery of Hesperidin/Chitosan Nanoparticles Suppresses Cytokine Storm Syndrome in a Mouse Model of Acute Lung Injury
Abstract
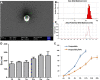
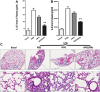
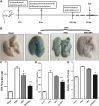
The cytokine storm or cytokine storm syndrome (CSS) is associated with high mortality in patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), for example following sepsis or infectious diseases including COVID-19. However, there are no effective treatments for CSS-associated ALI or ALI/ARDS. Thus, there remains an urgent need to develop effective drugs and therapeutic strategies against CSS and ALI/ARDS. Nasal and inhaled drug delivery methods represent a promising strategy in the treatment of inflammatory lung disease as a result of their ability to improve drug delivery to lungs. Improving the nasal mucosa absorption of poorly water-soluble drugs with poor mucosa bioavailability to a therapeutically effective level is another promising strategy in the fight against ALI/ARDS. Here, chitosan nanoparticles loaded with hesperidin (HPD/NPs) were developed for nasal delivery of the anti-inflammatory HPD compound to inflammatory lungs. In vitro and in vivo, HPD/NPs exhibited enhanced cellular uptake in the inflammatory microenvironment compared with free HPD. In a mouse model of inflammatory lung disease, the HPD/NPs markedly inhibited lung injury as evidenced by reduced inflammatory cytokine levels and suppressed vascular permeability compared with free HPD. Collectively, our study demonstrates that nasal delivery of HPD/NPs suppresses CSS and ALI/ARDS in a murine model of inflammatory lung disease, and that nanoparticle-based treatment strategies with anti-inflammatory effects could be used to reduce CSS and ALI in patients with inflammatory lung injury.
Keywords: chitosan nanoparticle; cytokine storm syndrome; hesperidin; lung inflammation; nasal drug delivery.
Copyright © 2021 Jin, Zhao, Lan, Zhou, Mai, Wang, Ding, Zhang, Pi, Evans and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
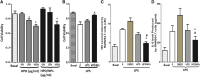


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

