Cynandione A Alleviates Neuropathic Pain Through α7-nAChR-Dependent IL-10/β-Endorphin Signaling Complexes
- PMID: 33584292
- PMCID: PMC7873367
- DOI: 10.3389/fphar.2020.614450
Cynandione A Alleviates Neuropathic Pain Through α7-nAChR-Dependent IL-10/β-Endorphin Signaling Complexes
Abstract
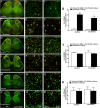
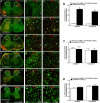
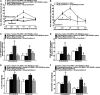
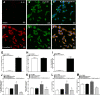
Cynandione A, an acetophenone isolated from Cynanchum Wilfordii Radix, exhibits antineuropathic pain effect. This study further explored the target molecule and signaling mechanisms underlying cynandione-A-induced antineuropathic pain. Intrathecal injection of cynandione A significantly attenuated mechanical allodynia in neuropathic rats and substantially increased spinal expression of IL-10 and β-endorphin but not dynorphin A. Cynandione A treatment also enhanced expression of IL-10 and β-endorphin but not α7 nicotinic acetylcholine receptors (nAChRs) in cultured microglia. The IL-10 antibody attenuated cynandione-A-induced spinal or microglial gene expression of β-endorphin and mechanical allodynia, whereas the β-endorphin antiserum blocked cynandione-A-induced mechanical antiallodynia but not spinal or microglial IL-10 gene expression. The α7 nAChR antagonist methyllycaconitine significantly reduced cynandione-A-induced mechanical antiallodynia and spinal or microglial expression of IL-10 and β-endorphin. Furthermore, cynandione A stimulated microglial phosphorylation of PKA, p38, and CREB in an α7-nAChR-dependent manner, and treatment with their inhibitors attenuated cynandione-A-induced mechanical antiallodynia and spinal or microglial expression of IL-10 and β-endorphin. In addition, cynandione A stimulated spinal phosphorylation of the transcription factor STAT3, which was inhibited by methyllycaconitine, the PKA activation inhibitor or IL-10 antibody. The STAT3 inhibitor NSC74859 also abolished cynandione-A-induced mechanical antiallodynia and spinal expression of β-endorphin. These findings suggest that cynandione A suppresses neuropathic pain through α7-nAChR-dependent IL-10/β-endorphin signaling pathway in spinal microglia.
Keywords: cynandione A; interleukin-10; microglia; neuropathic pain; α7 nicotinic acetylcholine receptor; β-endorphin.
Copyright © 2021 Han, Yin, Wang, Liu, Ao and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
IL-10/β-Endorphin-Mediated Neuroimmune Modulation on Microglia during Antinociception.Brain Sci. 2023 May 12;13(5):789. doi: 10.3390/brainsci13050789. Brain Sci. 2023. PMID: 37239261 Free PMC article. Review.
-
The spinal microglial IL-10/β-endorphin pathway accounts for cinobufagin-induced mechanical antiallodynia in bone cancer pain following activation of α7-nicotinic acetylcholine receptors.J Neuroinflammation. 2020 Feb 29;17(1):75. doi: 10.1186/s12974-019-1616-z. J Neuroinflammation. 2020. PMID: 32113469 Free PMC article.
-
Cynandione A attenuates neuropathic pain through p38β MAPK-mediated spinal microglial expression of β-endorphin.Brain Behav Immun. 2017 May;62:64-77. doi: 10.1016/j.bbi.2017.02.005. Epub 2017 Feb 9. Brain Behav Immun. 2017. PMID: 28189715
-
Cynandione A and PHA-543613 inhibit inflammation and stimulate macrophageal IL-10 expression following α7 nAChR activation.Biochem Pharmacol. 2021 Aug;190:114600. doi: 10.1016/j.bcp.2021.114600. Epub 2021 May 13. Biochem Pharmacol. 2021. PMID: 33992630
-
Regulation by Nicotinic Acetylcholine Receptors of Microglial Glutamate Transporters: Role of Microglia in Neuroprotection.2018 Apr 4. In: Akaike A, Shimohama S, Misu Y, editors. Nicotinic Acetylcholine Receptor Signaling in Neuroprotection [Internet]. Singapore: Springer; 2018. Chapter 5. 2018 Apr 4. In: Akaike A, Shimohama S, Misu Y, editors. Nicotinic Acetylcholine Receptor Signaling in Neuroprotection [Internet]. Singapore: Springer; 2018. Chapter 5. PMID: 31314410 Free Books & Documents. Review.
Cited by
-
IL-10/β-Endorphin-Mediated Neuroimmune Modulation on Microglia during Antinociception.Brain Sci. 2023 May 12;13(5):789. doi: 10.3390/brainsci13050789. Brain Sci. 2023. PMID: 37239261 Free PMC article. Review.
-
Targeting α7 nicotinic acetylcholine receptors for chronic pain.Front Mol Neurosci. 2022 Sep 30;15:970040. doi: 10.3389/fnmol.2022.970040. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36245927 Free PMC article. Review.
-
Trifluoro-Icaritin Ameliorates Neuroinflammation Against Complete Freund's Adjuvant-Induced Microglial Activation by Improving CB2 Receptor-Mediated IL-10/β-endorphin Signaling in the Spinal Cord of Rats.J Neuroimmune Pharmacol. 2024 Oct 10;19(1):53. doi: 10.1007/s11481-024-10152-8. J Neuroimmune Pharmacol. 2024. PMID: 39387998
-
The Role of Alpha-7 Nicotinic Acetylcholine Receptors in Pain: Potential Therapeutic Implications.Curr Neuropharmacol. 2025;23(2):129-144. doi: 10.2174/1570159X22666240528161117. Curr Neuropharmacol. 2025. PMID: 38808717 Free PMC article. Review.
-
Pulsed Electromagnetic Fields Protect Against Brain Ischemia by Modulating the Astrocytic Cholinergic Anti-inflammatory Pathway.Cell Mol Neurobiol. 2023 Apr;43(3):1301-1317. doi: 10.1007/s10571-022-01251-2. Epub 2022 Jul 13. Cell Mol Neurobiol. 2023. PMID: 35831547 Free PMC article.
References
-
- Apryani E., Ali U., Wang Z. Y., Wu H. Y., Mao X. F., Ahmad K. A., et al. (2020). The spinal microglial IL-10/β-endorphin pathway accounts for cinobufagin-induced mechanical antiallodynia in bone cancer pain following activation of α7-nicotinic acetylcholine receptors. J. Neuroinflammation 17 (1), 75 10.1186/s12974-019-1616-z - DOI - PMC - PubMed
-
- Arredondo J., Chernyavsky A. I., Jolkovsky D. L., Pinkerton K. E., Grando S. A. (2006). Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocyte. Faseb. J. 20 (12), 2093–2101. 10.1096/fj.06-6191com - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

