Triumeq Increases Excitability of Pyramidal Neurons in the Medial Prefrontal Cortex by Facilitating Voltage-Gated Ca2+ Channel Function
- PMID: 33584297
- PMCID: PMC7876243
- DOI: 10.3389/fphar.2020.617149
Triumeq Increases Excitability of Pyramidal Neurons in the Medial Prefrontal Cortex by Facilitating Voltage-Gated Ca2+ Channel Function
Abstract
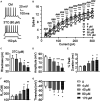
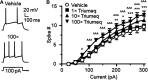
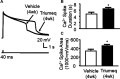
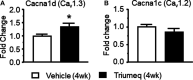
Combination antiretroviral therapy (cART) suppresses HIV-1 replication, improves immune function, and prolongs the life of people living with HIV (PLWH). However, cART also induces neurotoxicity that could complicate HIV-induced neurodegeneration while reduce its therapeutic efficacy in treating HIV/AIDS. Triumeq is a first-line cART regimen, which is co-formulated by three antiretroviral drugs (ARVs), lamivudine (3TC), abcavir (ABC), and dolutegravir (DTG). Little is known about potential side effects of ARVs on the brain (including those co-formulating Triumeq), and their mechanisms impacting neuronal activity. We assessed acute (in vitro) and chronic (in vivo) effects of Triumeq and co-formulating ARVs on pyramidal neurons in rat brain slices containing the medial prefrontal cortex (mPFC) using patch-clamp recording approaches. We found that acute Triumeq or 3TC in vitro significantly increased firing of mPFC neurons in a concentration- and time-dependent manner. This neuronal hyperactivity was associated with enhanced Ca2+ influx through voltage-gated Ca2+ channels (VGCCs). Additionally, chronic treatment with Triumeq in vivo for 4 weeks (4 wks) also significantly increased firing and Ca2+ influx via VGCCs in mPFC neurons, which was not shown after 2 wks treatment. Such mPFC neuronal hyperexcitability was not found after 4 weeks treatments of individual ARVs. Further, chronic Triumeq exposure in vivo significantly enhanced mRNA expression of low voltage-activated (LVA) L-type Ca2+ channels (Cav1.3 L-channels), while changes in high voltage-activated (HVA) Cav1.2 L-channels were not observed. Collectively, these novel findings demonstrate that chronic cART induces hyperexcitability of mPFC pyramidal neurons by abnormally promoting VGCC overactivation/overexpression of VGCCs (including, but may not limited to, LVA-Cav1.3 L-channels), which could complicate HIV-induced neurotoxicity, and ultimately may contribute to HIV-associated neurocognitive disorders (HAND) in PLWH. Determining additional target(s) of cART in mPFC pyramidal neurons may help to improve the therapeutic strategies by minimizing the side effects of cART for treating HIV/AIDS.
Keywords: CART; HIV-1; calcium dysregulation; electrophysiology; hyperactivity; neurodegenerative disease; neurotoxicity; voltage-gated calcium channel.
Copyright © 2021 Chen, Al-Harthi and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aouri M., Barcelo C., Ternon B., Cavassini M., Anagnostopoulos A., Yerly S., et al. (2016). In Vivo profiling and distribution of known and novel phase I and phase II metabolites of efavirenz in plasma, urine, and cerebrospinal fluid. Drug Metab. Dispos. 44, 151–161. 10.1124/dmd.115.065839 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

