Loss of FGFR3 Delays Acute Myeloid Leukemogenesis by Programming Weakly Pathogenic CD117-Positive Leukemia Stem-Like Cells
- PMID: 33584313
- PMCID: PMC7879375
- DOI: 10.3389/fphar.2020.632809
Loss of FGFR3 Delays Acute Myeloid Leukemogenesis by Programming Weakly Pathogenic CD117-Positive Leukemia Stem-Like Cells
Abstract
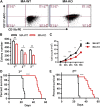
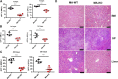
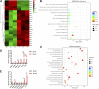
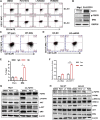
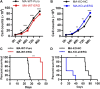
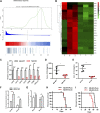
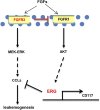
Chemotherapeutic patients with leukemia often relapse and produce drug resistance due to the existence of leukemia stem cells (LSCs). Fibroblast growth factor receptor 3 (FGFR3) signaling mediates the drug resistance of LSCs in chronic myeloid leukemia (CML). However, the function of FGFR3 in acute myeloid leukemia (AML) is less understood. Here, we identified that the loss of FGFR3 reprograms MLL-AF9 (MA)-driven murine AML cells into weakly pathogenic CD117-positive leukemia stem-like cells by activating the FGFR1-ERG signaling pathway. FGFR3 deletion significantly inhibits AML cells engraftment in vivo and extends the survival time of leukemic mice. FGFR3 deletion sharply decreased the expression of chemokines and the prolonged survival time in mice receiving FGFR3-deficient MA cells could be neutralized by overexpression of CCL3. Here we firstly found that FGFR3 had a novel regulatory mechanism for the stemness of LSCs in AML, and provided a promising anti-leukemia approach by interrupting FGFR3.
Keywords: CCL3 chemokine; FGFR1; erg; fibroblast growth factor receptor 3; leukemia (acute myeloid); stem-like cancer cells.
Copyright © 2021 Guo, Ran, Sun, Zhou, Yang, Zhang, Pang and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Atg5-dependent autophagy contributes to the development of acute myeloid leukemia in an MLL-AF9-driven mouse model.Cell Death Dis. 2016 Sep 8;7(9):e2361. doi: 10.1038/cddis.2016.264. Cell Death Dis. 2016. PMID: 27607576 Free PMC article.
-
Phosphoinositide-dependent kinase 1 regulates leukemia stem cell maintenance in MLL-AF9-induced murine acute myeloid leukemia.Biochem Biophys Res Commun. 2015 Apr 17;459(4):692-8. doi: 10.1016/j.bbrc.2015.03.007. Epub 2015 Mar 11. Biochem Biophys Res Commun. 2015. PMID: 25769952
-
[Expression of a tumor related gene fgfr3 mRNA in leukemic cells and its clinical significance].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008 Aug;16(4):738-41. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008. PMID: 18718050 Chinese.
-
[Progress in the studies of acute myelogenous leukemia stem cell].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2003 Oct;11(5):549-52. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2003. PMID: 14575558 Review. Chinese.
-
Targeting BMP signaling in the bone marrow microenvironment of myeloid leukemia.Biochem Soc Trans. 2020 Apr 29;48(2):411-418. doi: 10.1042/BST20190223. Biochem Soc Trans. 2020. PMID: 32167132 Review.
Cited by
-
Production of recombinant human long-acting IL-18 binding protein: inhibitory effect on ulcerative colitis in mice.Appl Microbiol Biotechnol. 2023 Dec;107(23):7135-7150. doi: 10.1007/s00253-023-12806-8. Epub 2023 Sep 28. Appl Microbiol Biotechnol. 2023. PMID: 37768347
-
Germline Variant Burden Warrants Universal Genetic Testing in Pediatric Myeloid Leukemia.medRxiv [Preprint]. 2025 Jul 30:2025.07.29.25332166. doi: 10.1101/2025.07.29.25332166. medRxiv. 2025. PMID: 40766138 Free PMC article. Preprint.
-
PPFIA1-targeting miR-181a mimic and saRNA overcome imatinib resistance in BCR-ABL1-independent chronic myeloid leukemia by suppressing leukemia stem cell regeneration.Mol Ther Nucleic Acids. 2023 May 4;32:729-742. doi: 10.1016/j.omtn.2023.04.026. eCollection 2023 Jun 13. Mol Ther Nucleic Acids. 2023. PMID: 37234746 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

