A Longitudinal Pilot Study on Cognition and Cerebral Hemodynamics in a Mouse Model of Preeclampsia Superimposed on Hypertension: Looking at Mothers and Their Offspring
- PMID: 33584345
- PMCID: PMC7878560
- DOI: 10.3389/fphys.2021.611984
A Longitudinal Pilot Study on Cognition and Cerebral Hemodynamics in a Mouse Model of Preeclampsia Superimposed on Hypertension: Looking at Mothers and Their Offspring
Abstract
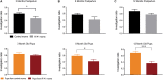
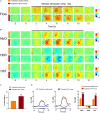
Preeclampsia is a common hypertensive disorder in pregnant women and whose causes and consequences have focused primarily on cardiovascular outcomes on the mother and offspring, often without taking into consideration the possible effects on the brain. One possible cause of preeclampsia has been attributed to alterations in the renin-angiotensin system, which has also been linked to cognitive decline. In this pilot study, we use a transgenic mouse model that chronically overexpresses human angiotensinogen and renin (R+A+ mice) that displayed characteristics of preeclampsia such as proteinuria during gestation. Offspring of these mothers as well as from control mothers were also examined. We were primarily interested in detecting whether cognitive deficits were present in the mothers and offspring in the long term and used a spatial learning and memory task as well as an object recognition task at three timepoints: 3, 8, and 12 months post-partum or post-natal, while measuring blood pressure and performing urine analysis after each timepoint. While we did not find significant deficits in preeclamptic mothers at the later timepoints, we did observe negative consequences in the pups of R+A+ mice that coincided with hemodynamic alterations whereby pups had higher whisker-evoked oxygenated hemoglobin levels and increased cerebral blood flow responses compared to control pups. Our study provides validation of this preeclampsia mouse model for future studies to decipher the underlying mechanisms of long-term cognitive deficits found in offspring.
Keywords: cerebral hemodynamics; cerebrovascular function; cognition; preeclampsia; renin—angiotensin—aldosterone system.
Copyright © 2021 Trigiani, Lecrux, Royea, Lavoie, Lesage, Pilote and Hamel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
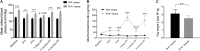

References
-
- Bokslag A., van Weissenbruch M., Mol B. W., de Groot C. J. (2016). Preeclampsia; short and long-term consequences for mother and neonate. Early Hum. Dev. 102 47–50. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

