Deep Brain Stimulation Initiative: Toward Innovative Technology, New Disease Indications, and Approaches to Current and Future Clinical Challenges in Neuromodulation Therapy
- PMID: 33584498
- PMCID: PMC7876228
- DOI: 10.3389/fneur.2020.597451
Deep Brain Stimulation Initiative: Toward Innovative Technology, New Disease Indications, and Approaches to Current and Future Clinical Challenges in Neuromodulation Therapy
Abstract
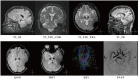
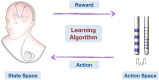
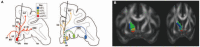
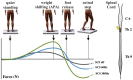
Deep brain stimulation (DBS) is one of the most important clinical therapies for neurological disorders. DBS also has great potential to become a great tool for clinical neuroscience research. Recently, the National Engineering Laboratory for Neuromodulation at Tsinghua University held an international Deep Brain Stimulation Initiative workshop to discuss the cutting-edge technological achievements and clinical applications of DBS. We specifically addressed new clinical approaches and challenges in DBS for movement disorders (Parkinson's disease and dystonia), clinical application toward neurorehabilitation for stroke, and the progress and challenges toward DBS for neuropsychiatric disorders. This review highlighted key developments in (1) neuroimaging, with advancements in 3-Tesla magnetic resonance imaging DBS compatibility for exploration of brain network mechanisms; (2) novel DBS recording capabilities for uncovering disease pathophysiology; and (3) overcoming global healthcare burdens with online-based DBS programming technology for connecting patient communities. The successful event marks a milestone for global collaborative opportunities in clinical development of neuromodulation to treat major neurological disorders.
Keywords: MRI compatibility; deep brain stimulation; depression; gait disability; neuromoxdulation.
Copyright © 2021 Sui, Tian, Ko, Wang, Jia, Horn, De Ridder, Choi, Bari, Wang, Hamani, Baker, Machado, Aziz, Fonoff, Kühn, Bergman, Sanger, Liu, Haber and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Evolving Applications, Technological Challenges and Future Opportunities in Neuromodulation: Proceedings of the Fifth Annual Deep Brain Stimulation Think Tank.Front Neurosci. 2018 Jan 24;11:734. doi: 10.3389/fnins.2017.00734. eCollection 2017. Front Neurosci. 2018. PMID: 29416498 Free PMC article.
-
Proceedings of the Seventh Annual Deep Brain Stimulation Think Tank: Advances in Neurophysiology, Adaptive DBS, Virtual Reality, Neuroethics and Technology.Front Hum Neurosci. 2020 Mar 27;14:54. doi: 10.3389/fnhum.2020.00054. eCollection 2020. Front Hum Neurosci. 2020. PMID: 32292333 Free PMC article.
-
Proceedings of the 11th Annual Deep Brain Stimulation Think Tank: pushing the forefront of neuromodulation with functional network mapping, biomarkers for adaptive DBS, bioethical dilemmas, AI-guided neuromodulation, and translational advancements.Front Hum Neurosci. 2024 Feb 21;18:1320806. doi: 10.3389/fnhum.2024.1320806. eCollection 2024. Front Hum Neurosci. 2024. PMID: 38450221 Free PMC article.
-
Potential indications for deep brain stimulation in neurological disorders: an evolving field.Eur J Neurol. 2018 Mar;25(3):434-e30. doi: 10.1111/ene.13548. Epub 2018 Feb 1. Eur J Neurol. 2018. PMID: 29266596 Review.
-
Deep brain stimulation for movement disorders: 2015 and beyond.Curr Opin Neurol. 2015 Aug;28(4):423-36. doi: 10.1097/WCO.0000000000000226. Curr Opin Neurol. 2015. PMID: 26110808 Review.
Cited by
-
Efficacy and safety of deep brain stimulation in mesencephalic locomotor region for motor function in patients with post-stroke hemiplegia: a study protocol for a multi-center double-blind crossover randomized controlled trial.Front Neurol. 2024 Aug 13;15:1355104. doi: 10.3389/fneur.2024.1355104. eCollection 2024. Front Neurol. 2024. PMID: 39193146 Free PMC article.
-
Novel Pharmacotherapies in Parkinson's Disease.Neurotox Res. 2021 Aug;39(4):1381-1390. doi: 10.1007/s12640-021-00375-5. Epub 2021 May 18. Neurotox Res. 2021. PMID: 34003454 Free PMC article. Review.
-
The ventral capsule and ventral striatum-Stereotactic targets for the management of treatment-resistant depression. A systematic literature review.Front Psychiatry. 2023 Oct 20;14:1100609. doi: 10.3389/fpsyt.2023.1100609. eCollection 2023. Front Psychiatry. 2023. PMID: 37928918 Free PMC article.
-
Bioelectric Potential in Next-Generation Organoids: Electrical Stimulation to Enhance 3D Structures of the Central Nervous System.Front Cell Dev Biol. 2022 May 17;10:901652. doi: 10.3389/fcell.2022.901652. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35656553 Free PMC article. Review.
-
Deep brain stimulation in globus pallidus internus travels to thalamus and subthalamic nuclei along physiological pathways.Front Neurosci. 2025 Jul 24;19:1592689. doi: 10.3389/fnins.2025.1592689. eCollection 2025. Front Neurosci. 2025. PMID: 40778353 Free PMC article.
References
-
- Henderson JM, Tkach J, Phillips M, Baker K, Shellock FG, Rezai AR. Permanent neurological deficit related to magnetic resonance imaging in a patient with implanted deep brain stimulation electrodes for Parkinson's disease: case report. Neurosurgery. (2005) 57:E1063. 10.1227/01.NEU.0000180810.16964.3E - DOI - PubMed
-
- IEC 60601-2-33:2010+AMD1:2013+AMD2:2015 CSV: Medical electrical equipment - Part 2-33: Particular requirements for the basic safety and essential performance of magnetic resonanceequipment for medical diagnosis .
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

