Functional Pituitary Networks in Vertebrates
- PMID: 33584547
- PMCID: PMC7873642
- DOI: 10.3389/fendo.2020.619352
Functional Pituitary Networks in Vertebrates
Abstract
The pituitary is a master endocrine gland that developed early in vertebrate evolution and therefore exists in all modern vertebrate classes. The last decade has transformed our view of this key organ. Traditionally, the pituitary has been viewed as a randomly organized collection of cells that respond to hypothalamic stimuli by secreting their content. However, recent studies have established that pituitary cells are organized in tightly wired large-scale networks that communicate with each other in both homo and heterotypic manners, allowing the gland to quickly adapt to changing physiological demands. These networks functionally decode and integrate the hypothalamic and systemic stimuli and serve to optimize the pituitary output into the generation of physiologically meaningful hormone pulses. The development of 3D imaging methods and transgenic models have allowed us to expand the research of functional pituitary networks into several vertebrate classes. Here we review the establishment of pituitary cell networks throughout vertebrate evolution and highlight the main perspectives and future directions needed to decipher the way by which pituitary networks serve to generate hormone pulses in vertebrates.
Keywords: evolution; networks; pituitary; plasticity; vertebrates.
Copyright © 2021 Santiago-Andres, Golan and Fiordelisio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


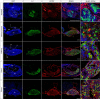

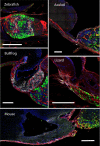
References
-
- Rathke H. Ueber die Entstehung der Glandula pituitaria. Arch Anat Physiol Sci Med (1838) 40:482–5.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

