Cryopreservation and Resuscitation of Natural Aquatic Prokaryotic Communities
- PMID: 33584565
- PMCID: PMC7877341
- DOI: 10.3389/fmicb.2020.597653
Cryopreservation and Resuscitation of Natural Aquatic Prokaryotic Communities
Abstract
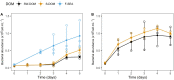
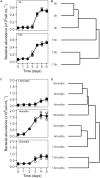
Experimental reproducibility in aquatic microbial ecology is critical to predict the dynamics of microbial communities. However, controlling the initial composition of naturally occurring microbial communities that will be used as the inoculum in experimental setups is challenging, because a proper method for the preservation of those communities is lacking. To provide a feasible method for preservation and resuscitation of natural aquatic prokaryote assemblages, we developed a cryopreservation procedure applied to natural aquatic prokaryotic communities. We studied the impact of inoculum size, processing time, and storage time on the success of resuscitation. We further assessed the effect of different growth media supplemented with dissolved organic matter (DOM) prepared from naturally occurring microorganisms on the recovery of the initially cryopreserved communities obtained from two sites that have contrasting trophic status and environmental heterogeneity. Our results demonstrated that the variability of the resuscitation process among replicates decreased with increasing inoculum size. The degree of similarity between initial and resuscitated communities was influenced by both the growth medium and origin of the community. We further demonstrated that depending on the inoculum source, 45-72% of the abundant species in the initially natural microbial communities could be detected as viable cells after cryopreservation. Processing time and long-term storage up to 12 months did not significantly influence the community composition after resuscitation. However, based on our results, we recommend keeping handling time to a minimum and ensure identical incubation conditions for repeated resuscitations from cryo-preserved aliquots at different time points. Given our results, we recommend cryopreservation as a promising tool to advance experimental research in the field of microbial ecology.
Keywords: aquatic environments; community composition; complex microbial communities; cryobiology; cryopreservation; culture; experimental microbiology; microbial ecology.
Copyright © 2021 Rain-Franco, de Moraes and Beier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Aminot A., Kérouel R. (2007). Dosage Automatique des Nutriments Dans les Eaux Marines: Méthodes en Flux Continu. Versailles: Editions Quae.
-
- Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Statist. Softw. 67 1–48. 10.18637/jss.v067.i01 - DOI
-
- Bell T., Gessner M. O., Griffiths R. I., McLaren J., Morin P. J., van der Heijden M., et al. (2009). “Microbial biodiversity and ecosystem functioning under controlled conditions and in the wild,” in Biodiversity, Ecosystem Functioning, and Human Wellbeing: An Ecological and Economic Perspective, eds Naeem S., Bunker D. E., Hector A., Loreau M., Perrings C. (Oxford: Oxford University Press; ).
LinkOut - more resources
Full Text Sources
Other Literature Sources

