Size Matters: Biological and Food Safety Relevance of Leaf Damage for Colonization of Escherichia coli O157:H7 gfp
- PMID: 33584570
- PMCID: PMC7873480
- DOI: 10.3389/fmicb.2020.608086
Size Matters: Biological and Food Safety Relevance of Leaf Damage for Colonization of Escherichia coli O157:H7 gfp
Abstract
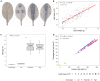
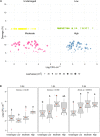
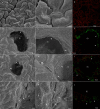
This study examined the biological and food safety relevance of leaf lesions for potential invasion of food pathogens into the plant tissue (internalization). This was done by determining the role of artificial leaf damage in terms of damaged leaf area on proliferation of E. coli O157:H7 gfp+. In a two-factorial experiment, unwashed fresh baby leaf spinach (Spinacia oleracea L.) was subjected to four damage levels (undamaged, low, moderate, high damage; factor 1) and three incubation intervals (0, 1, 2 days post-inoculation; factor 2). Individual leaves were immersed for 15 s in a suspension loaded with E. coli O157:H7 gfp+ (106 CFU × mL-1). The leaves were analyzed individually using image analysis tools to quantify leaf area and number and size of lesions, and using confocal laser scanning and scanning electron microscopy to visualize leaf lesions and presence of the introduced E. coli strain on and within the leaf tissue. Prevalence of E. coli O157:H7 gfp+ was assessed using a culture-dependent technique. The results showed that size of individual lesions and damaged leaf area affected depth of invasion into plant tissue, dispersal to adjacent areas, and number of culturable E. coli O157:H7 gfp+ directly after inoculation. Differences in numbers of the inoculant retrieved from leaf macerate evened out from 2 days post-inoculation, indicating rapid proliferation during the first day post-inoculation. Leaf weight was a crucial factor, as lighter spinach leaves (most likely younger leaves) were more prone to harbor E. coli O157:H7 gfp+, irrespective of damage level. At the high inoculum density used, the risk of consumers' infection was almost 100%, irrespective of incubation duration or damage level. Even macroscopically intact leaves showed a high risk for infection. These results suggest that the risk to consumers is correlated with how early in the food chain the leaves are contaminated, and the degree of leaf damage. These findings should be taken into account in different steps of leafy green processing. Further attention should be paid to the fate of viable, but non-culturable, shiga-toxigenic E. coli on and in ready-to-eat leafy vegetables.
Keywords: enterohemorrhagic E. coli; food safety; internalization; leafy vegetables; lesions; risk assessment; shiga-toxigenic E. coli; spinach (Spinacia oleracea L.).
Copyright © 2021 Mulaosmanovic, Windstam, Vågsholm and Alsanius.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Plant species affects establishment of Escherichia coli O157:H7 gfp+ on leafy vegetables.J Appl Microbiol. 2019 Jul;127(1):292-305. doi: 10.1111/jam.14299. Epub 2019 Jun 3. J Appl Microbiol. 2019. PMID: 31054164
-
Internalization of Escherichia coli O157:H7 gfp+ in rocket and Swiss chard baby leaves as affected by abiotic and biotic damage.Lett Appl Microbiol. 2017 Jul;65(1):35-41. doi: 10.1111/lam.12742. Epub 2017 May 30. Lett Appl Microbiol. 2017. PMID: 28397273
-
Effect of spinach cultivar and bacterial adherence factors on survival of Escherichia coli O157:H7 on spinach leaves.J Food Prot. 2013 Nov;76(11):1829-37. doi: 10.4315/0362-028X.JFP-12-556. J Food Prot. 2013. PMID: 24215684
-
Role of curli and cellulose expression in adherence of Escherichia coli O157:H7 to spinach leaves.Foodborne Pathog Dis. 2012 Feb;9(2):160-7. doi: 10.1089/fpd.2011.1020. Foodborne Pathog Dis. 2012. PMID: 22315954
-
Survival and colonization of Escherichia coli O157:H7 on spinach leaves as affected by inoculum level and carrier, temperature and relative humidity.J Appl Microbiol. 2011 Dec;111(6):1465-72. doi: 10.1111/j.1365-2672.2011.05175.x. Epub 2011 Nov 1. J Appl Microbiol. 2011. PMID: 21988171
References
-
- Allende A., McEvoy J., Tao Y., Luo Y. (2009). Antimicrobial effect of acidified sodium chlorite, sodium chlorite, sodium hypochlorite, and citric acid on Escherichia coli O157:H7 and natural microflora of fresh-cut cilantro. Food Control 20 230–234. 10.1016/j.foodcont.2008.05.009 - DOI
-
- Allende A., Selma M. V., López-Gálvez F., Villaescusa R., Gil M. I. (2008). Role of commercial sanitizers and washing systems on epiphytic microorganisms and sensory quality of fresh-cutescarole and lettuce. Postharvest Biol. Technol. 49 155–163. 10.1016/j.postharvbio.2007.12.010 - DOI
-
- Angle J. S., Gagliardi J. V., McIntosh M. S., Levin M. A. (1996). “Enumeration and expression of bacterial counts in the rhizosphere,” in Soil Biochemistry, eds Stolzky G., Bollag J.-M. (New York, NY: Marcel Dekker Inc; ), 233–251.
-
- Ariffin S. H., Gkatzionis K., Bakalis S. (2017). Leaf injury and its effect towards shelf-life and quality of ready-to-eat (RTE) spinach. Energy Procedia 123 105–112. 10.1016/j.egypro.2017.07.265 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

