The Prospective Synergy of Antitubercular Drugs With NAD Biosynthesis Inhibitors
- PMID: 33584600
- PMCID: PMC7873932
- DOI: 10.3389/fmicb.2020.634640
The Prospective Synergy of Antitubercular Drugs With NAD Biosynthesis Inhibitors
Abstract
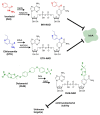
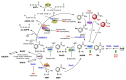
Given the upsurge of drug-resistant tuberculosis worldwide, there is much focus on developing novel drug combinations allowing shorter treatment duration and a lower toxicity profile. Nicotinamide adenine dinucleotide (NAD) biosynthesis targeting is acknowledged as a promising strategy to combat drug-susceptible, drug-resistant, and latent tuberculosis (TB) infections. In this review, we describe the potential synergy of NAD biosynthesis inhibitors with several TB-drugs in prospective novel combination therapy. Despite not directly targeting the essential NAD cofactor's biosynthesis, several TB prodrugs either require a NAD biosynthesis enzyme to be activated or form a toxic chemical adduct with NAD(H) itself. For example, pyrazinamide requires the action of nicotinamidase (PncA), often referred to as pyrazinamidase, to be converted into its active form. PncA is an essential player in NAD salvage and recycling. Since most pyrazinamide-resistant strains are PncA-defective, a combination with downstream NAD-blocking molecules may enhance pyrazinamide activity and possibly overcome the resistance mechanism. Isoniazid, ethionamide, and delamanid form NAD adducts in their active form, partly perturbing the redox cofactor metabolism. Indeed, NAD depletion has been observed in Mycobacterium tuberculosis (Mtb) during isoniazid treatment, and activation of the intracellular NAD phosphorylase MbcT toxin potentiates its effect. Due to the NAD cofactor's crucial role in cellular energy production, additional synergistic correlations of NAD biosynthesis blockade can be envisioned with bedaquiline and other drugs targeting energy-metabolism in mycobacteria. In conclusion, future strategies targeting NAD metabolism in Mtb should consider its potential synergy with current and other forthcoming TB-drugs.
Keywords: NAD biosynthesis inhibition; delamanid; ethionamide; isoniazid; nicotinamide; pyrazinamide; toxin-antitoxin system; tuberculosis.
Copyright © 2021 Rohde and Sorci.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Study of mechanism of interaction of truncated isoniazid-nicotinamide adenine dinucleotide adduct against multiple enzymes of Mycobacterium tuberculosis by a computational approach.Int J Mycobacteriol. 2015 Dec;4(4):276-83. doi: 10.1016/j.ijmyco.2015.06.006. Epub 2015 Jul 15. Int J Mycobacteriol. 2015. PMID: 26964808
-
Pyrazinamide: the importance of uncovering the mechanisms of action in mycobacteria.Expert Rev Anti Infect Ther. 2015 May;13(5):593-603. doi: 10.1586/14787210.2015.1021784. Epub 2015 Mar 6. Expert Rev Anti Infect Ther. 2015. PMID: 25746054 Review.
-
API TB Consensus Guidelines 2006: Management of pulmonary tuberculosis, extra-pulmonary tuberculosis and tuberculosis in special situations.J Assoc Physicians India. 2006 Mar;54:219-34. J Assoc Physicians India. 2006. PMID: 16800350
-
Delamanid Kills Dormant Mycobacteria In Vitro and in a Guinea Pig Model of Tuberculosis.Antimicrob Agents Chemother. 2017 May 24;61(6):e02402-16. doi: 10.1128/AAC.02402-16. Print 2017 Jun. Antimicrob Agents Chemother. 2017. PMID: 28373190 Free PMC article.
-
Mutations Associated with Pyrazinamide Resistance in Mycobacterium tuberculosis: A Review and Update.Curr Microbiol. 2022 Oct 8;79(11):348. doi: 10.1007/s00284-022-03032-y. Curr Microbiol. 2022. PMID: 36209317 Review.
Cited by
-
Gallein and isoniazid act synergistically to attenuate Mycobacterium tuberculosis growth in human macrophages.bioRxiv [Preprint]. 2024 Jan 10:2024.01.10.574965. doi: 10.1101/2024.01.10.574965. bioRxiv. 2024. PMID: 38260681 Free PMC article. Preprint.
-
Novel Prodrug Strategies for the Treatment of Tuberculosis.Chem Asian J. 2024 Dec 2;19(23):e202400944. doi: 10.1002/asia.202400944. Epub 2024 Oct 24. Chem Asian J. 2024. PMID: 39179514 Free PMC article. Review.
-
Synthesis, Biological, and Computational Evaluations of Conformationally Restricted NAD-Mimics as Discriminant Inhibitors of Human NMN-Adenylyltransferase Isozymes.Pharmaceuticals (Basel). 2024 Jun 6;17(6):739. doi: 10.3390/ph17060739. Pharmaceuticals (Basel). 2024. PMID: 38931406 Free PMC article.
-
Gallein potentiates isoniazid's ability to suppress Mycobacterium tuberculosis growth.Front Microbiol. 2024 Apr 15;15:1369763. doi: 10.3389/fmicb.2024.1369763. eCollection 2024. Front Microbiol. 2024. PMID: 38690363 Free PMC article.
-
The Reductionist Conundrum of an "Updated" Definition of Extensively Drug-Resistant Tuberculosis.Am J Respir Crit Care Med. 2021 Sep 15;204(6):629-631. doi: 10.1164/rccm.202106-1516ED. Am J Respir Crit Care Med. 2021. PMID: 34375572 Free PMC article. No abstract available.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

