Inactivation of Pepper Mild Mottle Virus in Water by Cold Atmospheric Plasma
- PMID: 33584622
- PMCID: PMC7877120
- DOI: 10.3389/fmicb.2021.618209
Inactivation of Pepper Mild Mottle Virus in Water by Cold Atmospheric Plasma
Abstract
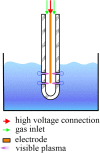
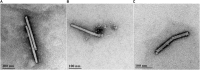
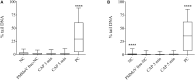
Water scarcity is one of the greatest threats for human survival and quality of life, and this is increasingly contributing to the risk of human, animal and plant infections due to waterborne viruses. Viruses are transmitted through polluted water, where they can survive and cause infections even at low concentrations. Plant viruses from the genus Tobamovirus are highly mechanically transmissible, and cause considerable damage to important crops, such as tomato. The release of infective tobamoviruses into environmental waters has been reported, with the consequent risk for arid regions, where these waters are used for irrigation. Virus inactivation in water is thus very important and cold atmospheric plasma (CAP) is emerging in this field as an efficient, safe, and sustainable alternative to classic waterborne virus inactivation methods. In the present study we evaluated CAP-mediated inactivation of pepper mild mottle virus (PMMoV) in water samples. PMMoV is a very resilient water-transmissible tobamovirus that can survive transit through the human digestive tract. The efficiency of PMMoV inactivation was characterized for infectivity and virion integrity, and at the genome level, using test plant infectivity assays, transmission electron microscopy, and molecular methods, respectively. Additionally, the safety of CAP treatment was determined by testing the cytotoxic and genotoxic properties of CAP-treated water on the HepG2 cell line. 5-min treatment with CAP was sufficient to inactivate PMMoV without introducing any cytotoxic or genotoxic effects in the in-vitro cell model system. These data on inactivation of such stable waterborne virus, PMMoV, will encourage further examination of CAP as an alternative for treatment of potable and irrigation waters, and even for other water sources, with emphasis on inactivation of various viruses including enteric viruses.
Keywords: cold atmospheric plasma; enteric viruses; pepper mild mottle virus; virus inactivation; water decontamination.
Copyright © 2021 Filipić, Dobnik, Tušek Žnidarič, Žegura, Štern, Primc, Mozetič, Ravnikar, Žel and Gutierrez Aguirre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Aboubakr H. A., Gangal U., Youssef M. M., Goyal S. M., Bruggeman P. J. (2016). Inactivation of virus in solution by cold atmospheric pressure plasma: identification of chemical inactivation pathways. J. Phys. D. Appl. Phys. 49 1–17.
-
- Aboubakr H. A., Sampedro Parra F., Collins J., Bruggeman P., Goyal S. M. (2020). Ìn situ inactivation of human norovirus GII.4 by cold plasma: ethidium monoazide (EMA)-coupled RT-qPCR underestimates virus reduction and fecal material suppresses inactivation. Food Microbiol. 85:103307. 10.1016/j.fm.2019.103307 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

