Complement Activation in Kidneys of Patients With COVID-19
- PMID: 33584662
- PMCID: PMC7878379
- DOI: 10.3389/fimmu.2020.594849
Complement Activation in Kidneys of Patients With COVID-19
Abstract
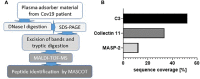
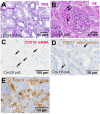
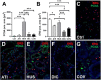
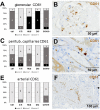
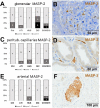
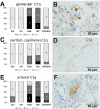
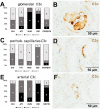
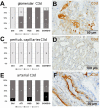
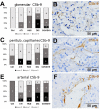
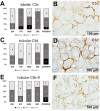
Most patients who became critically ill following infection with COVID-19 develop severe acute respiratory syndrome (SARS) attributed to a maladaptive or inadequate immune response. The complement system is an important component of the innate immune system that is involved in the opsonization of viruses but also in triggering further immune cell responses. Complement activation was seen in plasma adsorber material that clogged during the treatment of critically ill patients with COVID-19. Apart from the lung, the kidney is the second most common organ affected by COVID-19. Using immunohistochemistry for complement factors C1q, MASP-2, C3c, C3d, C4d, and C5b-9 we investigated the involvement of the complement system in six kidney biopsies with acute kidney failure in different clinical settings and three kidneys from autopsy material of patients with COVID-19. Renal tissue was analyzed for signs of renal injury by detection of thrombus formation using CD61, endothelial cell rarefaction using the marker E-26 transformation specific-related gene (ERG-) and proliferation using proliferating cell nuclear antigen (PCNA)-staining. SARS-CoV-2 was detected by in situ hybridization and immunohistochemistry. Biopsies from patients with hemolytic uremic syndrome (HUS, n = 5), severe acute tubular injury (ATI, n = 7), zero biopsies with disseminated intravascular coagulation (DIC, n = 7) and 1 year protocol biopsies from renal transplants (Ctrl, n = 7) served as controls. In the material clogging plasma adsorbers used for extracorporeal therapy of patients with COVID-19 C3 was the dominant protein but collectin 11 and MASP-2 were also identified. SARS-CoV-2 was sporadically present in varying numbers in some biopsies from patients with COVID-19. The highest frequency of CD61-positive platelets was found in peritubular capillaries and arteries of COVID-19 infected renal specimens as compared to all controls. Apart from COVID-19 specimens, MASP-2 was detected in glomeruli with DIC and ATI. In contrast, the classical pathway (i.e. C1q) was hardly seen in COVID-19 biopsies. Both C3 cleavage products C3c and C3d were strongly detected in renal arteries but also occurs in glomerular capillaries of COVID-19 biopsies, while tubular C3d was stronger than C3c in biopsies from COVID-19 patients. The membrane attack complex C5b-9, demonstrating terminal pathway activation, was predominantly deposited in COVID-19 biopsies in peritubular capillaries, renal arterioles, and tubular basement membrane with similar or even higher frequency compared to controls. In conclusion, various complement pathways were activated in COVID-19 kidneys, the lectin pathway mainly in peritubular capillaries and in part the classical pathway in renal arteries whereas the alternative pathway seem to be crucial for tubular complement activation. Therefore, activation of the complement system might be involved in the worsening of renal injury. Complement inhibition might thus be a promising treatment option to prevent deregulated activation and subsequent collateral tissue injury.
Keywords: COVID-19; complement—immunological term; endothelial injury; kidney; lectin pathway activation.
Copyright © 2021 Pfister, Vonbrunn, Ries, Jäck, Überla, Lochnit, Sheriff, Herrmann, Büttner-Herold, Amann and Daniel.
Conflict of interest statement
Author AS was employed by the company Pentracor GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

