Irradiated Tumor Fibroblasts Avoid Immune Recognition and Retain Immunosuppressive Functions Over Natural Killer Cells
- PMID: 33584669
- PMCID: PMC7874190
- DOI: 10.3389/fimmu.2020.602530
Irradiated Tumor Fibroblasts Avoid Immune Recognition and Retain Immunosuppressive Functions Over Natural Killer Cells
Abstract
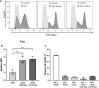
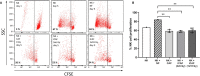
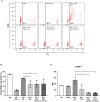
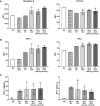
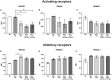
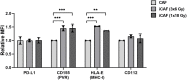
Recent studies have demonstrated that radiotherapy is able to induce anti-tumor immune responses in addition to mediating direct cytotoxic effects. Cancer-associated fibroblasts (CAFs) are central constituents of the tumor stroma and participate actively in tumor immunoregulation. However, the capacity of CAFs to influence immune responses in the context of radiotherapy is still poorly understood. This study was undertaken to determine whether ionizing radiation alters the CAF-mediated immunoregulatory effects on natural killer (NK) cells. CAFs were isolated from freshly resected non-small cell lung cancer tissues, while NK cells were prepared from peripheral blood of healthy donors. Functional assays to study NK cell immune activation included proliferation rates, expression of cell surface markers, secretion of immunomodulators, cytotoxic assays, as well as production of intracellular activation markers such as perforin and granzyme B. Our data show that CAFs inhibit NK cell activation by reducing their proliferation rates, the cytotoxic capacity, the extent of degranulation, and the surface expression of stimulatory receptors, while concomitantly enhancing surface expression of inhibitory receptors. Radiation delivered as single high-dose or in fractioned regimens did not reverse the immunosuppressive features exerted by CAFs over NK cells in vitro, despite triggering enhanced surface expression of several checkpoint ligands on irradiated CAFs. In summary, CAFs mediate noticeable immune inhibitory effects on cytokine-activated NK cells during co-culture in a donor-independent manner. However, ionizing radiation does not interfere with the CAF-mediated immunosuppressive effects.
Keywords: TME; cancer-associated fibroblasts; immune evasion; immunosuppression; immunotherapy; ionizing radiation; radiotherapy; tumor microenvironment.
Copyright © 2021 Yang, Lode, Berzaghi, Islam, Martinez-Zubiaurre and Hellevik.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

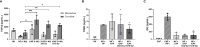
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

