Extracellular Adenosine Triphosphate (eATP) and Its Metabolite, Extracellular Adenosine (eAdo), as Opposing "Yin-Yang" Regulators of Nlrp3 Inflammasome in the Trafficking of Hematopoietic Stem/Progenitor Cells
- PMID: 33584673
- PMCID: PMC7878390
- DOI: 10.3389/fimmu.2020.603942
Extracellular Adenosine Triphosphate (eATP) and Its Metabolite, Extracellular Adenosine (eAdo), as Opposing "Yin-Yang" Regulators of Nlrp3 Inflammasome in the Trafficking of Hematopoietic Stem/Progenitor Cells
Abstract
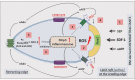
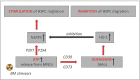
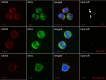
Nlrp3 inflammasome plays a pleiotropic role in hematopoietic cells. On the one hand, physiological activation of this intracellular protein complex is crucial to maintaining normal hematopoiesis and the trafficking of hematopoietic stem progenitor cells (HSPCs). On the other hand, its hyperactivation may lead to cell death by pyroptosis, and prolonged activity is associated with sterile inflammation of the BM and, as a consequence, with the HSPCs aging and origination of myelodysplasia and leukemia. Thus, we need to understand better this protein complex's actions to define the boundaries of its safety window and study the transition from being beneficial to being detrimental. As demonstrated, the Nlrp3 inflammasome is expressed and active both in HSPCs and in the non-hematopoietic cells that are constituents of the bone marrow (BM) microenvironment. Importantly, the Nlrp3 inflammasome responds to mediators of purinergic signaling, and while extracellular adenosine triphosphate (eATP) activates this protein complex, its metabolite extracellular adenosine (eAdo) has the opposite effect. In this review, we will discuss and focus on the physiological consequences of the balance between eATP and eAdo in regulating the trafficking of HSPCs in an Nlrp3 inflammasome-dependent manner, as seen during pharmacological mobilization from BM into peripheral blood (PB) and in the reverse mechanism of homing from PB to BM and engraftment. We propose that both mediators of purinergic signaling and the Nlrp3 inflammasome itself may become important therapeutic targets in optimizing the trafficking of HSPCs in clinical settings.
Keywords: NOD-like receptor family pyrin domain-containing protein 3 (Nlrp3) inflammasome; extracellular ATP; purinergic signaling; stem cell homing and engraftment; stem cell mobilization.
Copyright © 2021 Ratajczak and Kucia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

