TFH Cells Induced by Vaccination and Following SIV Challenge Support Env-Specific Humoral Immunity in the Rectal-Genital Tract and Circulation of Female Rhesus Macaques
- PMID: 33584682
- PMCID: PMC7876074
- DOI: 10.3389/fimmu.2020.608003
TFH Cells Induced by Vaccination and Following SIV Challenge Support Env-Specific Humoral Immunity in the Rectal-Genital Tract and Circulation of Female Rhesus Macaques
Abstract
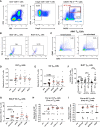
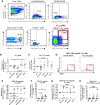
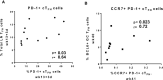
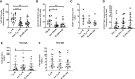
T follicular helper (TFH) cells are pivotal in lymph node (LN) germinal center (GC) B cell affinity maturation. Circulating CXCR5+ CD4+ T (cTFH) cells have supported memory B cell activation and broadly neutralizing antibodies in HIV controllers. We investigated the contribution of LN SIV-specific TFH and cTFH cells to Env-specific humoral immunity in female rhesus macaques following a mucosal Ad5hr-SIV recombinant priming and SIV gp120 intramuscular boosting vaccine regimen and following SIV vaginal challenge. TFH and B cells were characterized by flow cytometry. B cell help was evaluated in TFH-B cell co-cultures and by real-time PCR. Vaccination induced Env-specific TFH and Env-specific memory (ESM) B cells in LNs. LN Env-specific TFH cells post-priming and GC ESM B cells post-boosting correlated with rectal Env-specific IgA titers, and GC B cells at the same timepoints correlated with vaginal Env-specific IgG titers. Vaccination expanded cTFH cell responses, including CD25+ Env-specific cTFH cells that correlated negatively with vaginal Env-specific IgG titers but positively with rectal Env-specific IgA titers. Although cTFH cells post-2nd boost positively correlated with viral-loads following SIV challenge, cTFH cells of SIV-infected and protected macaques supported maturation of circulating B cells into plasma cells and IgA release in co-culture. Additionally, cTFH cells of naïve macaques promoted upregulation of genes associated with B cell proliferation, BCR engagement, plasma cell maturation, and antibody production, highlighting the role of cTFH cells in blood B cell maturation. Vaccine-induced LN TFH and GC B cells supported anti-viral mucosal immunity while cTFH cells provided B cell help in the periphery during immunization and after SIV challenge. Induction of TFH responses in blood and secondary lymphoid organs is likely desirable for protective efficacy of HIV vaccines.
Keywords: B cell help; SIV vaccine; TFH cells; humoral immunity; rhesus macaque.
Copyright © 2021 Helmold Hait, Hogge, Rahman, Hunegnaw, Mushtaq, Hoang and Robert-Guroff.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Early T Follicular Helper Cell Responses and Germinal Center Reactions Are Associated with Viremia Control in Immunized Rhesus Macaques.J Virol. 2019 Feb 5;93(4):e01687-18. doi: 10.1128/JVI.01687-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463978 Free PMC article.
-
Enhanced immunity and protective efficacy against SIVmac251 intrarectal challenge following ad-SIV priming by multiple mucosal routes and gp120 boosting in MPL-SE.Viral Immunol. 2005;18(1):236-43. doi: 10.1089/vim.2005.18.236. Viral Immunol. 2005. PMID: 15802969
-
Improved protection of rhesus macaques against intrarectal simian immunodeficiency virus SIV(mac251) challenge by a replication-competent Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinant priming/gp120 boosting regimen.J Virol. 2003 Aug;77(15):8354-65. doi: 10.1128/jvi.77.15.8354-8365.2003. J Virol. 2003. PMID: 12857905 Free PMC article.
-
Role of T Follicular Helper Cells in Viral Infections and Vaccine Design.Cells. 2025 Mar 29;14(7):508. doi: 10.3390/cells14070508. Cells. 2025. PMID: 40214462 Free PMC article. Review.
-
Gut Microbiome Homeostasis and the CD4 T- Follicular Helper Cell IgA Axis in Human Immunodeficiency Virus Infection.Front Immunol. 2021 Mar 19;12:657679. doi: 10.3389/fimmu.2021.657679. eCollection 2021. Front Immunol. 2021. PMID: 33815419 Free PMC article. Review.
Cited by
-
Long-Term Analysis of Pertussis Vaccine Immunity to Identify Potential Markers of Vaccine-Induced Memory Associated With Whole Cell But Not Acellular Pertussis Immunization in Mice.Front Immunol. 2022 Feb 8;13:838504. doi: 10.3389/fimmu.2022.838504. eCollection 2022. Front Immunol. 2022. PMID: 35211125 Free PMC article.
-
Gut Microbiome and Bile Acid Metabolism Induced the Activation of CXCR5+ CD4+ T Follicular Helper Cells to Participate in Neuromyelitis Optica Spectrum Disorder Recurrence.Front Immunol. 2022 Jan 20;13:827865. doi: 10.3389/fimmu.2022.827865. eCollection 2022. Front Immunol. 2022. PMID: 35126400 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

