Mechanisms Governing Immunotherapy Resistance in Pancreatic Ductal Adenocarcinoma
- PMID: 33584701
- PMCID: PMC7876239
- DOI: 10.3389/fimmu.2020.613815
Mechanisms Governing Immunotherapy Resistance in Pancreatic Ductal Adenocarcinoma
Abstract
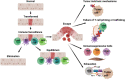
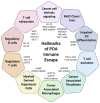
Pancreatic ductal adenocarcinoma (PDA) is a lethal malignancy with an overall 5-year survival rate of 10%. Disease lethality is due to late diagnosis, early metastasis and resistance to therapy, including immunotherapy. PDA creates a robust fibroinflammatory tumor microenvironment that contributes to immunotherapy resistance. While previously considered an immune privileged site, evidence demonstrates that in some cases tumor antigen-specific T cells infiltrate and preferentially accumulate in PDA and are central to tumor cell clearance and long-term remission. Nonetheless, PDA can rapidly evade an adaptive immune response using a myriad of mechanisms. Mounting evidence indicates PDA interferes with T cell differentiation into potent cytolytic effector T cells via deficiencies in naive T cell priming, inducing T cell suppression or promoting T cell exhaustion. Mechanistic research indicates that immunotherapy combinations that change the suppressive tumor microenvironment while engaging antigen-specific T cells is required for treatment of advanced disease. This review focuses on recent advances in understanding mechanisms limiting T cell function and current strategies to overcome immunotherapy resistance in PDA.
Keywords: PD-1; PD-L1; T cell; exhaustion; immunosuppression; immunotherapy; pancreatic cancer; pancreatic ductal adenocarcinoma.
Copyright © 2021 Schmiechen and Stromnes.
Conflict of interest statement
IS serves on the scientific advisory board for Luminary Therapeutics and Immunogenesis. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975-2016. Bethesda, MD: National Cancer Institute; (2019). Available at: https://seer.cancer.gov/csr/1975_2016/.
-
- Huang L, Jansen L, Balavarca Y, van der Geest L, Lemmens L, Van Eycken L, et al. Stratified survival of resected and overall pancreatic cancer patients in Europe and the USA in the early twenty-first century: a large, international population-based study Lei. BMC Med (2018) 16. 10.1186/s12916-018-1120-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

