Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases
- PMID: 33584709
- PMCID: PMC7873987
- DOI: 10.3389/fimmu.2020.617089
Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases
Abstract
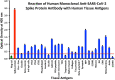
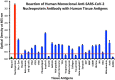
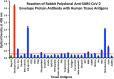
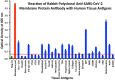
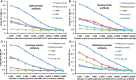
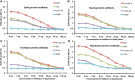
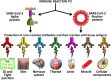
We sought to determine whether immune reactivity occurs between anti-SARS-CoV-2 protein antibodies and human tissue antigens, and whether molecular mimicry between COVID-19 viral proteins and human tissues could be the cause. We applied both human monoclonal anti-SARS-Cov-2 antibodies (spike protein, nucleoprotein) and rabbit polyclonal anti-SARS-Cov-2 antibodies (envelope protein, membrane protein) to 55 different tissue antigens. We found that SARS-CoV-2 antibodies had reactions with 28 out of 55 tissue antigens, representing a diversity of tissue groups that included barrier proteins, gastrointestinal, thyroid and neural tissues, and more. We also did selective epitope mapping using BLAST and showed similarities and homology between spike, nucleoprotein, and many other SARS-CoV-2 proteins with the human tissue antigens mitochondria M2, F-actin and TPO. This extensive immune cross-reactivity between SARS-CoV-2 antibodies and different antigen groups may play a role in the multi-system disease process of COVID-19, influence the severity of the disease, precipitate the onset of autoimmunity in susceptible subgroups, and potentially exacerbate autoimmunity in subjects that have pre-existing autoimmune diseases. Very recently, human monoclonal antibodies were approved for use on patients with COVID-19. The human monoclonal antibodies used in this study are almost identical with these approved antibodies. Thus, our results can establish the potential risk for autoimmunity and multi-system disorders with COVID-19 that may come from cross-reactivity between our own human tissues and this dreaded virus, and thus ensure that the badly-needed vaccines and treatments being developed for it are truly safe to use against this disease.
Keywords: COVID-19; SARS-CoV-2; autoimmunity; cross-reactivity; molecular mimicry.
Copyright © 2021 Vojdani, Vojdani and Kharrazian.
Conflict of interest statement
AV is the co-owner, CEO and employee of Immunosciences Lab., Inc. EV is the owner and employee of Regenera Medical, a private medical practice. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

