Persistent Systemic Inflammation in Patients With Severe Burn Injury Is Accompanied by Influx of Immature Neutrophils and Shifts in T Cell Subsets and Cytokine Profiles
- PMID: 33584717
- PMCID: PMC7879574
- DOI: 10.3389/fimmu.2020.621222
Persistent Systemic Inflammation in Patients With Severe Burn Injury Is Accompanied by Influx of Immature Neutrophils and Shifts in T Cell Subsets and Cytokine Profiles
Abstract
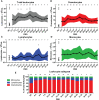
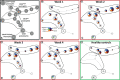
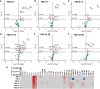
Severe burn injury causes local and systemic immune responses that can persist up to months, and can lead to systemic inflammatory response syndrome, organ damage and long-term sequalae such as hypertrophic scarring. To prevent these pathological conditions, a better understanding of the underlying mechanisms is essential. In this longitudinal study, we analyzed the temporal peripheral blood immune profile of 20 burn wound patients admitted to the intensive care by flow cytometry and secretome profiling, and compared this to data from 20 healthy subjects. The patient cohort showed signs of systemic inflammation and persistently high levels of pro-inflammatory soluble mediators, such as IL-6, IL-8, MCP-1, MIP-1β, and MIP-3α, were measured. Using both unsupervised and supervised flow cytometry techniques, we observed a continuous release of neutrophils and monocytes into the blood for at least 39 days. Increased numbers of immature neutrophils were present in peripheral blood in the first three weeks after injury (0.1-2.8 × 106/ml after burn vs. 5 × 103/ml in healthy controls). Total lymphocyte numbers did not increase, but numbers of effector T cells as well as regulatory T cells were increased from the second week onward. Within the CD4+ T cell population, elevated numbers of CCR4+CCR6- and CCR4+CCR6+ cells were found. Altogether, these data reveal that severe burn injury induced a persistent innate inflammatory response, including a release of immature neutrophils, and shifts in the T cell composition toward an overall more pro-inflammatory phenotype, thereby continuing systemic inflammation and increasing the risk of secondary complications.
Keywords: burn injury; flow cytometry; immune response; inflammation; lymphocytes; monocytes; neutrophils; systemic.
Copyright © 2021 Mulder, Vlig, Boekema, Stoop, Pijpe, van Zuijlen, de Jong, van Cranenbroek, Joosten, Koenen and Ulrich.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

