The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease
- PMID: 33584724
- PMCID: PMC7878386
- DOI: 10.3389/fimmu.2020.622468
The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease
Abstract
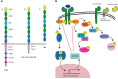
Leptin is a critical mediator of the immune response to changes in overall nutrition. Leptin is produced by adipocytes in proportion to adipose tissue mass and is therefore increased in obesity. Despite having a well-described role in regulating systemic metabolism and appetite, leptin displays pleiotropic actions, and it is now clear that leptin has a key role in influencing immune cell function. Indeed, many immune cells have been shown to respond to leptin directly via the leptin receptor, resulting in a largely pro-inflammatory phenotype. Understanding the role of adipose-tissue derived mediators in inflammation is critical to determining the pathophysiology of multiple obesity-associated diseases, such as type 2 diabetes, autoimmune disease, and infection. This review, therefore, focuses on the latest data regarding the role of leptin in modulating inflammation.
Keywords: adaptive immunity; adipose tissue; inflammation; leptin; obesity.
Copyright © 2021 Kiernan and MacIver.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Leptin and Leptin Signaling in Multiple Sclerosis: A Narrative Review.Neuromolecular Med. 2025 Feb 28;27(1):19. doi: 10.1007/s12017-025-08842-4. Neuromolecular Med. 2025. PMID: 40019662 Free PMC article. Review.
-
An update on leptin as immunomodulator.Expert Rev Clin Immunol. 2014 Sep;10(9):1165-70. doi: 10.1586/1744666X.2014.942289. Epub 2014 Aug 7. Expert Rev Clin Immunol. 2014. PMID: 25098336 Review.
-
Adipose tissue, adipokines, and inflammation.J Allergy Clin Immunol. 2005 May;115(5):911-9; quiz 920. doi: 10.1016/j.jaci.2005.02.023. J Allergy Clin Immunol. 2005. PMID: 15867843 Review.
-
Recent advances in the relationship between obesity, inflammation, and insulin resistance.Eur Cytokine Netw. 2006 Mar;17(1):4-12. Eur Cytokine Netw. 2006. PMID: 16613757 Review.
-
Role of leptin in the activation of immune cells.Mediators Inflamm. 2010;2010:568343. doi: 10.1155/2010/568343. Epub 2010 Mar 23. Mediators Inflamm. 2010. PMID: 20368778 Free PMC article. Review.
Cited by
-
Evidence That Peripheral Leptin Resistance in Omental Adipose Tissue and Liver Correlates with MASLD in Humans.Int J Mol Sci. 2024 Jun 11;25(12):6420. doi: 10.3390/ijms25126420. Int J Mol Sci. 2024. PMID: 38928125 Free PMC article.
-
Anti-Obesity Activity of Sanghuangporus vaninii by Inhibiting Inflammation in Mice Fed a High-Fat Diet.Nutrients. 2024 Jul 6;16(13):2159. doi: 10.3390/nu16132159. Nutrients. 2024. PMID: 38999906 Free PMC article.
-
Obese visceral fat tissue inflammation: from protective to detrimental?BMC Med. 2022 Dec 27;20(1):494. doi: 10.1186/s12916-022-02672-y. BMC Med. 2022. PMID: 36575472 Free PMC article. Review.
-
Low Intensity Pulsed Ultrasounds Modulate Adipose Stem Cells Differentiation.Stem Cell Rev Rep. 2025 Aug;21(6):1760-1775. doi: 10.1007/s12015-025-10896-7. Epub 2025 May 23. Stem Cell Rev Rep. 2025. PMID: 40408066 Free PMC article.
-
Leptin antagonism attenuates hypertension and renal injury in an experimental model of autoimmune disease.Clin Sci (Lond). 2023 Dec 14;137(23):1771-1785. doi: 10.1042/CS20230924. Clin Sci (Lond). 2023. PMID: 38031726 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

