Metabolic Modeling of the C3-CAM Continuum Revealed the Establishment of a Starch/Sugar-Malate Cycle in CAM Evolution
- PMID: 33584741
- PMCID: PMC7874232
- DOI: 10.3389/fpls.2020.573197
Metabolic Modeling of the C3-CAM Continuum Revealed the Establishment of a Starch/Sugar-Malate Cycle in CAM Evolution
Abstract
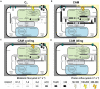
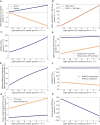
The evolution of Crassulacean acid metabolism (CAM) is thought to be along a C3-CAM continuum including multiple variations of CAM such as CAM cycling and CAM idling. Here, we applied large-scale constraint-based modeling to investigate the metabolism and energetics of plants operating in C3, CAM, CAM cycling, and CAM idling. Our modeling results suggested that CAM cycling and CAM idling could be potential evolutionary intermediates in CAM evolution by establishing a starch/sugar-malate cycle. Our model analysis showed that by varying CO2 exchange during the light period, as a proxy of stomatal conductance, there exists a C3-CAM continuum with gradual metabolic changes, supporting the notion that evolution of CAM from C3 could occur solely through incremental changes in metabolic fluxes. Along the C3-CAM continuum, our model predicted changes in metabolic fluxes not only through the starch/sugar-malate cycle that is involved in CAM photosynthetic CO2 fixation but also other metabolic processes including the mitochondrial electron transport chain and the tricarboxylate acid cycle at night. These predictions could guide engineering efforts in introducing CAM into C3 crops for improved water use efficiency.
Keywords: CAM cycling; CAM evolution; CAM idling; crassulacean acid metabolism; flux balance analysis; metabolic modeling.
Copyright © 2021 Tay, Odang and Cheung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Cheung C. Y. M., Poolman M. G., Fell D. A., Ratcliffe R. G., Sweetlove L. J. (2014). A Diel Flux Balance Model Captures Interactions between Light and Dark Metabolism during Day-Night Cycles in C3 and Crassulacean Acid Metabolism Leaves. Plant Physiol. 165 917–929. 10.1104/pp.113.234468 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

