Improving Nitrogen Use Efficiency Through Overexpression of Alanine Aminotransferase in Rice, Wheat, and Barley
- PMID: 33584777
- PMCID: PMC7875890
- DOI: 10.3389/fpls.2021.628521
Improving Nitrogen Use Efficiency Through Overexpression of Alanine Aminotransferase in Rice, Wheat, and Barley
Abstract
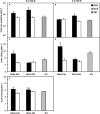
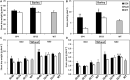
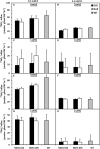
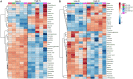
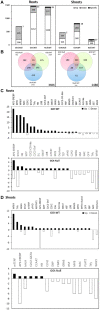
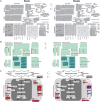
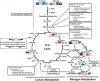
Nitrogen is an essential nutrient for plants, but crop plants are inefficient in the acquisition and utilization of applied nitrogen. This often results in producers over applying nitrogen fertilizers, which can negatively impact the environment. The development of crop plants with more efficient nitrogen usage is, therefore, an important research goal in achieving greater agricultural sustainability. We utilized genetically modified rice lines over-expressing a barley alanine aminotransferase (HvAlaAT) to help characterize pathways which lead to more efficient use of nitrogen. Under the control of a stress-inducible promoter OsAnt1, OsAnt1:HvAlaAT lines have increased above-ground biomass with little change to both nitrate and ammonium uptake rates. Based on metabolic profiles, carbon metabolites, particularly those involved in glycolysis and the tricarboxylic acid (TCA) cycle, were significantly altered in roots of OsAnt1:HvAlaAT lines, suggesting higher metabolic turnover. Moreover, transcriptomic data revealed that genes involved in glycolysis and TCA cycle were upregulated. These observations suggest that higher activity of these two processes could result in higher energy production, driving higher nitrogen assimilation, consequently increasing biomass production. Other potential mechanisms contributing to a nitrogen-use efficient phenotype include involvements of phytohormonal responses and an alteration in secondary metabolism. We also conducted basic growth studies to evaluate the effect of the OsAnt1:HvAlaAT transgene in barley and wheat, which the transgenic crop plants increased seed production under controlled environmental conditions. This study provides comprehensive profiling of genetic and metabolic responses to the over-expression of AlaAT and unravels several components and pathways which contribute to its nitrogen-use efficient phenotype.
Keywords: RNAseq; alanine aminotransferase; carbohydrate metabolism; nitrogen use efficiency; transgenic cereals.
Copyright © 2021 Tiong, Sharma, Sampath, MacKenzie, Watanabe, Metot, Lu, Skinner, Lu, Kridl, Baumann, Heuer, Kaiser and Okamoto.
Conflict of interest statement
JK was a named inventor on United States Patents Nitrogen-Efficient Monocot Plants US 8642840 and 8288611, and Arcadia Biosciences has exclusively licensed these patents and others concerning the NUE technology described. JK, WS, YL, and ZL are employees of Arcadia Biosciences, Inc. and as such Arcadia partially funded the work described. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ashhurst D. E. (2001). Chitin and chitinases. Cell Biochem. Funct. 19 228–228. 10.1002/cbf.916 - DOI
-
- Ashraf M., Foolad M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59 206–216. 10.1016/j.envexpbot.2005.12.006 - DOI
-
- Beatty P. H., Carroll R. T., Shrawat A. K., Guevara D., Good A. G. (2013). Physiological analysis of nitrogen-efficient rice overexpressing alanine aminotransferase under different N regimes. Bot. Botanique 91 866–883. 10.1139/cjb-2013-0171 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

