Wheat Can Access Phosphorus From Algal Biomass as Quickly and Continuously as From Mineral Fertilizer
- PMID: 33584779
- PMCID: PMC7879783
- DOI: 10.3389/fpls.2021.631314
Wheat Can Access Phosphorus From Algal Biomass as Quickly and Continuously as From Mineral Fertilizer
Abstract
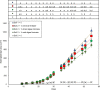
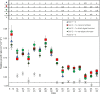
Algae can efficiently take up excess nutrients from waterways, making them a valuable resource potentially capable of replacing synthesized and mined fertilizers for agriculture. The capacity of algae to fertilize crops has been quantified, but it is not known how the algae-derived nutrients become available to plants. We aimed to address this question: what are the temporal dynamics of plant growth responses to algal biomass? to better propose mechanisms by which plants acquire nutrients from algal biomass and thereby study and promote those processes in future agricultural applications. Data from various sources were transformed and used to reconstruct the nutrient release from the algae Chlorella vulgaris and subsequent uptake by wheat (Triticum aestivum L.) (as reported in Schreiber et al., 2018). Plants had received 0.1x or 1x dried algae or wet algae, or zero, 0.1x or 1x mineral fertilizer calculated from agricultural practices for P application and grown to 55 days in three soils. Contents of P and other nutrients acquired from algae were as high as from mineral fertilizer, but varied based on moisture content and amount of algae applied to soils (by 55 days after sowing plants with 1x mineral fertilizer and 1x dried algae had 5.6 mg P g DWshoot; 2.2-fold more than those with 0 or 0.1x mineral fertilizer, 0.1x dried algae and wet algae, and 1x wet algae). Absolute and relative leaf area growth and estimated P uptake rates showed similar dynamics, indicating that wheat acquires P from algae quickly. A model proposes that algal fertilizer promotes wheat growth after rapid transformation in soil to inorganic nutrients. We conclude theoretically that phosphorus from algal biomass is available to wheat seedlings upon its application and is released gradually over time with minor differences related to moisture content on application. The growth and P uptake kinetics hint at nutrient forms, including N, and biomass stimulation worthy of research to further exploit algae in sustainable agriculture practices. Temporal resolved phenotype analyses in combination with a mass-balance approach is helpful for understanding resource uptake from recycled and biofertilizer sources by plants.
Keywords: algae fertilizer; mass balance; phosphorus; plant growth; resource efficiency; wheat.
Copyright © 2021 Mau, Kant, Walker, Kuchendorf, Schrey, Roessner and Watt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alobwede E., Leake J. R., Pandhal J. (2019). Circular economy fertilization: testing micro and macro algal species as soil improvers and nutrient sources for crop production in greenhouse and field conditions. Geoderma 334 113–123. 10.1016/j.geoderma.2018.07.049 - DOI
-
- Aoki S., Miyachi S. (1964). Chromatographic analyses of acid-soluble polyphosphates in chlorella cells. Plant Cell Physiol. 5 241–250. 10.1093/oxfordjournals.pcp.a079038 - DOI
-
- Bloem E., Albihn A., Elving J., Hermann L., Lehmann L., Sarvi M., et al. (2017). Contamination of organic nutrient sources with potentially toxic elements, antibiotics and pathogen microorganisms in relation to P fertilizer potential and treatment options for the production of sustainable fertilizers: a review. Sci. Total Environ. 60 225–242. 10.1016/j.scitotenv.2017.06.274 - DOI - PubMed
-
- De Wit C. T. (1992). Resource use efficiency in agriculture. Agric. Syst. 40 125–151.
LinkOut - more resources
Full Text Sources
Other Literature Sources

