Stellate Genes and the piRNA Pathway in Speciation and Reproductive Isolation of Drosophila melanogaster
- PMID: 33584811
- PMCID: PMC7874207
- DOI: 10.3389/fgene.2020.610665
Stellate Genes and the piRNA Pathway in Speciation and Reproductive Isolation of Drosophila melanogaster
Abstract
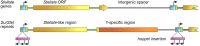
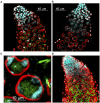
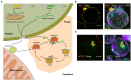
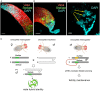
One of the main conditions of the species splitting from a common precursor lineage is the prevention of a gene flow between diverging populations. The study of Drosophila interspecific hybrids allows to reconstruct the speciation mechanisms and to identify hybrid incompatibility factors that maintain post-zygotic reproductive isolation between closely related species. The regulation, evolution, and maintenance of the testis-specific Ste-Su(Ste) genetic system in Drosophila melanogaster is the subject of investigation worldwide. X-linked tandem testis-specific Stellate genes encode proteins homologous to the regulatory β-subunit of protein kinase CK2, but they are permanently repressed in wild-type flies by the piRNA pathway via piRNAs originating from the homologous Y-linked Su(Ste) locus. Derepression of Stellate genes caused by Su(Ste) piRNA biogenesis disruption leads to the accumulation of crystalline aggregates in spermatocytes, meiotic defects and male sterility. In this review we summarize current data about the origin, organization, evolution of the Ste-Su(Ste) system, and piRNA-dependent regulation of Stellate expression. The Ste-Su(Ste) system is fixed only in the D. melanogaster genome. According to our hypothesis, the acquisition of the Ste-Su(Ste) system by a part of the ancient fly population appears to be the causative factor of hybrid sterility in crosses of female flies with males that do not carry Y-linked Su(Ste) repeats. To support this scenario, we have directly demonstrated Stellate derepression and the corresponding meiotic disorders in the testes of interspecies hybrids between D. melanogaster and D. mauritiana. This finding embraces our hypothesis about the contribution of the Ste-Su(Ste) system and the piRNA pathway to the emergence of reproductive isolation of D. melanogaster lineage from initial species.
Keywords: Drosophila; Stellate genes; hybrid sterility; piRNA pathway; reproductive isolation.
Copyright © 2021 Adashev, Kotov, Bazylev, Shatskikh, Aravin and Olenina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
piRNA silencing contributes to interspecies hybrid sterility and reproductive isolation in Drosophila melanogaster.Nucleic Acids Res. 2019 May 7;47(8):4255-4271. doi: 10.1093/nar/gkz130. Nucleic Acids Res. 2019. PMID: 30788506 Free PMC article.
-
Peculiarities of piRNA-mediated post-transcriptional silencing of Stellate repeats in testes of Drosophila melanogaster.Nucleic Acids Res. 2009 Jun;37(10):3254-63. doi: 10.1093/nar/gkp167. Epub 2009 Mar 24. Nucleic Acids Res. 2009. PMID: 19321499 Free PMC article.
-
Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline.Curr Biol. 2001 Jul 10;11(13):1017-27. doi: 10.1016/s0960-9822(01)00299-8. Curr Biol. 2001. PMID: 11470406
-
[Molecular evolution of tandem heterochromatic repeats in connection with their function in the genome of Drosophila melanogaster].Genetika. 2002 Jun;38(6):710-8. Genetika. 2002. PMID: 12138770 Review. Russian.
-
Paralogous stellate and Su(Ste) repeats: evolution and ability to silence a reporter gene.Genetica. 2000;109(1-2):131-40. doi: 10.1023/a:1026596419250. Genetica. 2000. PMID: 11293788 Review.
Cited by
-
miRNA/siRNA-directed pathway to produce noncoding piRNAs from endogenous protein-coding regions ensures Drosophila spermatogenesis.Sci Adv. 2023 Jul 21;9(29):eadh0397. doi: 10.1126/sciadv.adh0397. Epub 2023 Jul 19. Sci Adv. 2023. PMID: 37467338 Free PMC article.
-
A comparative roadmap of PIWI-interacting RNAs across seven species reveals insights into de novo piRNA-precursor formation in mammals.Cell Rep. 2024 Oct 22;43(10):114777. doi: 10.1016/j.celrep.2024.114777. Epub 2024 Sep 19. Cell Rep. 2024. PMID: 39302833 Free PMC article.
-
An introduction to PIWI-interacting RNAs (piRNAs) in the context of metazoan small RNA silencing pathways.RNA Biol. 2022 Jan;19(1):1094-1102. doi: 10.1080/15476286.2022.2132359. RNA Biol. 2022. PMID: 36217279 Free PMC article. Review.
-
piRNA associates with immune diseases.Cell Commun Signal. 2024 Jun 28;22(1):347. doi: 10.1186/s12964-024-01724-5. Cell Commun Signal. 2024. PMID: 38943141 Free PMC article. Review.
-
Small RNA-mediated suppression of sex chromosome meiotic conflicts during Drosophila male gametogenesis.Biochem Soc Trans. 2025 Feb 6;53(1):BST20240344. doi: 10.1042/BST20240344. Biochem Soc Trans. 2025. PMID: 39918264 Free PMC article. Review.
References
-
- Ackerman P., Glover C. V., Osheroff N. (1988). Phosphorylation of DNA topoisomerase II in vivo and in total homogenates of Drosophila Kc cells. The role of casein kinase II. J. Biol. Chem. 263, 12653–12660. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

