Early Treatment Targets for Predicting Long-term Dermatology Life Quality Index Response in Patients with Moderate-to-Severe Plaque Psoriasis: A Post-hoc Analysis from a Long-term Clinical Study
- PMID: 33584952
- PMCID: PMC7840086
Early Treatment Targets for Predicting Long-term Dermatology Life Quality Index Response in Patients with Moderate-to-Severe Plaque Psoriasis: A Post-hoc Analysis from a Long-term Clinical Study
Abstract
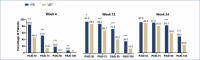
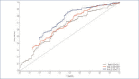
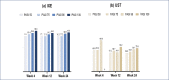
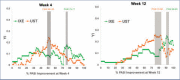
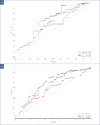
BACKGROUND: Rapid improvements in health-related quality of life (HRQoL) and psoriasis severity have been reported in patients treated with ixekizumab (IXE), an interleukin (IL)-17A antibody. OBJECTIVE: We assessed the relationship between early Psoriasis Area and Severity Index (PASI) response and long-term Dermatology Life Quality Index (DLQI) improvement in patients in the randomized clinical trial IXORA-S (NCT0256186) treated with IXE or IL-12/23 (ustekinumab [UST]). METHODS: The proportion of patients achieving DLQI (0,1), an outcome equivalent to the patient's skin condition having no impact on HRQoL after 52 weeks of IXE or UST by PASI response at Weeks 4, 12, and 24 was quantified. Optimal thresholds for PASI response by treatment to predict Week 52 DLQI (0,1) were calculated based on Youden's Index. RESULTS: Early and higher levels of skin clearance were associated with improved patient outcomes regardless of treatment. Patients treated with IXE achieved faster and more pronounced PASI response than patients treated with UST. The optimal thresholds at Weeks 4, 12, and 24 for predicting DLQI (0,1) at Week 52 were ~PASI 75 for IXE versus ~PASI 50 for UST at Week 4, PASI 90 for IXE versus PASI 75 for UST at Week 12, and ~PASI 100 for IXE versus ~PASI 90 for UST at Week 24. Among patients achieving these thresholds, the probability of achieving a DLQI (0,1) was significantly higher. CONCLUSION: Earlier and higher levels of skin clearance are associated with improved patient outcomes over the long term, regardless of treatment.
Keywords: Dermatology Life Quality Index; Psoriasis Area and Severity Index; ixekizumab; psoriasis.
Copyright © 2020. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
FUNDING:The study was sponsored by Eli Lilly and Company. DISCLOSURES:Professor Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Centocor, Covagen, Eli Lilly and Company, Forward Pharma, Fresenius Medical Care, Galapagos NV, GlaxoSmithKline, Janssen-Cilag, Kyowa Kirin, Leo, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, and Xenoport. Dr. Puig has received grants/research supports or participation in clinical trials (paid to Institution). Drs. Zhu, Burge, Shrom, Dong, Shen, and Mallbris are employees and minor stockholders of Eli Lilly and Company.
Figures

References
-
- Kimble AB. Unmet needs in the management of plaque psoriasis. Managed Care. 2009;18(Suppl 1):1–7.
-
- Stern RS, Nijstan T, Feldman SR et al. Psoriasis is common, carries a substantial burden event when non extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136–139. - PubMed
-
- Revicki DA, Willian MK, Menter A et al. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate-to-severe psoriasis. Dermatology. 2008;216:260–270. - PubMed
-
- Mattei P, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients with biological therapies. J Eur Acad Dermatol Venereol. 2014;28:333–337. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
