A Split-face, Controlled Study to Assess the Compatibility of Tretinoin 0.05% Acne Lotion with Facial Foundation Makeup
- PMID: 33584959
- PMCID: PMC7840090
A Split-face, Controlled Study to Assess the Compatibility of Tretinoin 0.05% Acne Lotion with Facial Foundation Makeup
Abstract
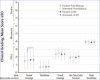
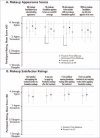
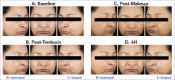
OBJECTIVE: This study was conducted to assess compatibility of tretinoin 0.05% acne lotion with foundation makeup. DESIGN: This was a single-center, evaluator-blinded, randomized, controlled clinical trial. Participants were randomized to apply tretinoin 0.05% lotion to either the right or left side of the face before applying full-face foundation makeup. SETTING: Participants were enrolled at a single center in the United States. PARTICIPANTS: Female individuals aged 18 to 50 years who used facial foundation makeup ≥5 days per week were included. MEASUREMENTS: Investigator-assessed grading for foundation coverage and participant evaluations of makeup appearance were conducted at post-makeup application (Post-Makeup) and Hour 6 (6H) timepoints. Antera 3D® images were taken for skin texture roughness analysis and tolerability evaluations were performed at baseline, post-tretinoin application, Post-Makeup, and 6H timepoints. RESULTS: A total of 30 participants were enrolled and 29 completed the study. There were no significant differences between tretinoin treated and untreated sides for any outcomes of investigator-assessed grading of foundation (percentage coverage, blotchiness, overall coverage, skin tone evenness, visual smoothness). There was a small but statistically significant worsening in percent coverage at 6H versus Post-Makeup on the untreated side, but not the treated side. As rated by participants, even/full coverage and skin smoothness were significantly better on the tretinoin-lotion treated versus the untreated side. Three-dimensional imaging showed there were no significant differences in skin roughness between the treated and untreated sides. Participants reported overall satisfaction with the tretinoin lotion-treated side. CONCLUSION: Tretinoin 0.05% lotion did not interfere with facial makeup application or wearability and was well tolerated.
Keywords: Retinoid; acne; foundation; lotion; makeup; tretinoin.
Copyright © 2020. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
FUNDING:This study was funded by Ortho Dermatologics. DISCLOSURES:Bausch Health US, LLC is an affiliate of Bausch Health Companies Inc. Ortho Dermatologics is a division of Bausch Health US, LLC. Neal Bhatia has received honoraria and investigator grants from Bausch Health. Leon Kircik has acted as an investigator, advisor, speaker, and consultant for Ortho Dermatologics. Ava Shamban has received grants/ research support and/or consulting from Galderma, Merz, Endo, Brickell, B10, Revance, Teoxane, and Allergan. Varsha Bhatt and Radhakrishnan Pillai are employees of Bausch Health US, LLC and may hold stock and/or stock options in its parent company. Eric Guenin is an employee of Ortho Dermatologics and may hold stock and/or stock options in its parent company. Bausch Health US, LLC is an affiliate of Bausch Health Companies Inc. Ortho Dermatologics is a division of Bausch Health US, LLC.
Figures
Similar articles
-
Randomized, Observer-blind, Split-face Compatibility Study with Clindamycin Phosphate 1.2%/Benzoyl Peroxide 3.75% gel and Facial Foundation Makeup.J Clin Aesthet Dermatol. 2015 Sep;8(9):25-32. J Clin Aesthet Dermatol. 2015. PMID: 26430488 Free PMC article.
-
Vehicle Formulation Impacts Tolerability and Patient Preference: Comparison of Tretinoin Branded Lotion and Generic Cream.J Drugs Dermatol. 2022 Aug 1;21(8):875-880. doi: 10.36849/JDD.6945. J Drugs Dermatol. 2022. PMID: 35946981 Clinical Trial.
-
Tretinoin 0.05% Lotion for the Once-Daily Treatment of Moderate-to-Severe Acne Vulgaris: Impact of Gender and Race on Efficacy and Safety.J Drugs Dermatol. 2019 Nov 1;18(11):1128-1138. J Drugs Dermatol. 2019. PMID: 31741356 Clinical Trial.
-
Efficacy and Tolerability of a Novel Tretinoin 0.05% Lotion for the Once-Daily Treatment of Moderate or Severe Acne Vulgaris in Adult Females.J Drugs Dermatol. 2019 Nov 1;18(11):1147-1154. J Drugs Dermatol. 2019. PMID: 31741360 Clinical Trial.
-
Hygiene and emollient interventions for maintaining skin integrity in older people in hospital and residential care settings.Cochrane Database Syst Rev. 2020 Jan 23;1(1):CD011377. doi: 10.1002/14651858.CD011377.pub2. Cochrane Database Syst Rev. 2020. PMID: 32006460 Free PMC article.
Cited by
-
Effect of Makeup Use on Depressive Symptoms: An Open, Randomized and Controlled Trial.Dermatol Ther (Heidelb). 2024 Mar;14(3):777-791. doi: 10.1007/s13555-024-01128-w. Epub 2024 Mar 21. Dermatol Ther (Heidelb). 2024. PMID: 38509378 Free PMC article.
-
Acne treatment challenges - Recommendations of Latin American expert consensus.An Bras Dermatol. 2024 May-Jun;99(3):414-424. doi: 10.1016/j.abd.2023.09.001. Epub 2024 Feb 23. An Bras Dermatol. 2024. PMID: 38402012 Free PMC article. Review.
References
-
- Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(1):3–12. - PubMed
-
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41(4):577–580. - PubMed
-
- Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78(3):335–341. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
