Harnessing the Immune System Against Multiple Myeloma: Challenges and Opportunities
- PMID: 33585226
- PMCID: PMC7873734
- DOI: 10.3389/fonc.2020.606368
Harnessing the Immune System Against Multiple Myeloma: Challenges and Opportunities
Abstract
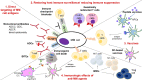
Multiple myeloma (MM) is an incurable malignancy of plasma cells that grow within a permissive bone marrow microenvironment (BMM). The bone marrow milieu supports the malignant transformation both by promoting uncontrolled proliferation and resistance to cell death in MM cells, and by hampering the immune response against the tumor clone. Hence, it is expected that restoring host anti-MM immunity may provide therapeutic benefit for MM patients. Already several immunotherapeutic approaches have shown promising results in the clinical setting. In this review, we outline recent findings demonstrating the potential advantages of targeting the immunosuppressive bone marrow niche to restore effective anti-MM immunity. We discuss different approaches aiming to boost the effector function of T cells and/or exploit innate or adaptive immunity, and highlight novel therapeutic opportunities to increase the immunogenicity of the MM clone. We also discuss the main challenges that hamper the efficacy of immune-based approaches, including intrinsic resistance of MM cells to activated immune-effectors, as well as the protective role of the immune-suppressive and inflammatory bone marrow milieu. Targeting mechanisms to convert the immunologically "cold" to "hot" MM BMM may induce durable immune responses, which in turn may result in long-lasting clinical benefit, even in patient subgroups with high-risk features and poor survival.
Keywords: challanges; immune system; immunotherapy; microenvironment; myeloma.
Copyright © 2021 Yamamoto, Amodio, Gulla and Anderson.
Conflict of interest statement
KA serves on advisory boards to Celgene, Millennium, Janssen, Sanofi, Bristol Myers Squibb, Gilead, Precision Biosciences, and Tolero and is a Scientific Founder of OncoPep andC4 Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewers SO and MC declared a past co-authorship with one of the authors, respectively, KA and NA to the handling editor.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

