Effect of Amine Functionalization of MOF Adsorbents for Enhanced CO2 Capture and Separation: A Molecular Simulation Study
- PMID: 33585395
- PMCID: PMC7873881
- DOI: 10.3389/fchem.2020.574622
Effect of Amine Functionalization of MOF Adsorbents for Enhanced CO2 Capture and Separation: A Molecular Simulation Study
Abstract
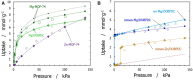
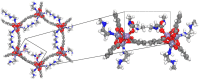
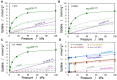
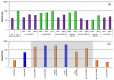
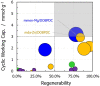
Different types of amine-functionalized MOF structures were analyzed in this work using molecular simulations in order to determine their potential for post-combustion carbon dioxide capture and separation. Six amine models -of different chain lengths and degree of substitution- grafted to the unsaturated metal sites of the M2(dobdc) MOF [and its expanded version, M2(dobpdc)] were evaluated, in terms of adsorption isotherms, selectivity, cyclic working capacity and regenerability. Good agreement between simulation results and available experimental data was obtained. Moreover, results show two potential structures with high cyclic working capacities if used for Temperature Swing Adsorption processes: mmen/Mg/DOBPDC and mda-Zn/DOBPDC. Among them, the -mmen functionalized structure has higher CO2 uptake and better cyclability (regenerability) for the flue gas mixtures and conditions studied. Furthermore, it is shown that more amine functional groups grafted on the MOFs and/or full functionalization of the metal centers do not lead to better CO2 separation capabilities due to steric hindrances. In addition, multiple alkyl groups bonded to the amino group yield a shift in the step-like adsorption isotherms in the larger pore structures, at a given temperature. Our calculations shed light on how functionalization can enhance gas adsorption via the cooperative chemi-physisorption mechanism of these materials, and how the materials can be tuned for desired adsorption characteristics.
Keywords: CO2 capture; MOF-74; Monte Carlo simulation; amine; chemisorption; functionalization; metal-organic frameworks.
Copyright © 2021 Bahamon, Anlu, Builes, Khaleel and Vega.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Alkhatib I. I. I., Pereira L. M. C., AlHajaj A., Vega L. F. (2020). Performance of non-aqueous amine hybrid solvents mixtures for CO2 capture: a study using a molecular-based model. J. CO2 Util. 35, 126–144. 10.1016/j.jcou.2019.09.010 - DOI
-
- Alonso G., Bahamon D., Keshavarz F., Giménez X., Gamallo P., Sayós R. (2018). Density functional theory-based adsorption isotherms for pure and flue gas mixtures on Mg-MOF-74. Application in CO2 capture swing adsorption processes. J. Phys. Chem. C 122, 3945–3957. 10.1021/acs.jpcc.8b00938 - DOI
-
- Arstad B., Fjellvåg H., Kongshaug K. O., Swang O., Blom R. (2008). Amine functionalised metal organic frameworks (MOFs) as adsorbents for carbon dioxide. Adsorption 14, 755–762. 10.1007/s10450-008-9137-6 - DOI
-
- Bahamon D., Abu-Zahra M. R. M., Vega L. F. (2019). Molecular simulations of carbon-based materials for selected CO2 separation and water treatment processes. Fluid Phase Eq. 492, 10–25. 10.1016/j.fluid.2019.03.014 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

