In vivo and Post-synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review
- PMID: 33585417
- PMCID: PMC7874203
- DOI: 10.3389/fbioe.2020.619266
In vivo and Post-synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review
Abstract
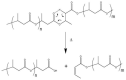
The transition toward "green" alternatives to petroleum-based plastics is driven by the need for "drop-in" replacement materials able to combine characteristics of existing plastics with biodegradability and renewability features. Promising alternatives are the polyhydroxyalkanoates (PHAs), microbial biodegradable polyesters produced by a wide range of microorganisms as carbon, energy, and redox storage material, displaying properties very close to fossil-fuel-derived polyolefins. Among PHAs, polyhydroxybutyrate (PHB) is by far the most well-studied polymer. PHB is a thermoplastic polyester, with very narrow processability window, due to very low resistance to thermal degradation. Since the melting temperature of PHB is around 170-180°C, the processing temperature should be at least 180-190°C. The thermal degradation of PHB at these temperatures proceeds very quickly, causing a rapid decrease in its molecular weight. Moreover, due to its high crystallinity, PHB is stiff and brittle resulting in very poor mechanical properties with low extension at break, which limits its range of application. A further limit to the effective exploitation of these polymers is related to their production costs, which is mostly affected by the costs of the starting feedstocks. Since the first identification of PHB, researchers have faced these issues, and several strategies to improve the processability and reduce brittleness of this polymer have been developed. These approaches range from the in vivo synthesis of PHA copolymers, to the enhancement of post-synthesis PHB-based material performances, thus the addition of additives and plasticizers, acting on the crystallization process as well as on polymer glass transition temperature. In addition, reactive polymer blending with other bio-based polymers represents a versatile approach to modulate polymer properties while preserving its biodegradability. This review examines the state of the art of PHA processing, shedding light on the green and cost-effective tailored strategies aimed at modulating and optimizing polymer performances. Pioneering examples in this field will be examined, and prospects and challenges for their exploitation will be presented. Furthermore, since the establishment of a PHA-based industry passes through the designing of cost-competitive production processes, this review will inspect reported examples assessing this economic aspect, examining the most recent progresses toward process sustainability.
Keywords: bio-based network; biopolymer; plasticizer; polyhydroxybutyrate; reactive processing.
Copyright © 2021 Turco, Santagata, Corrado, Pezzella and Di Serio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aeschelmann F., Carus M. (2017). Bio-Based Building Blocks and Polymers. Global Capacities. nova-Institut GmbH. Available online at: http://www.bio-based.eu/reports/
-
- Alexandre M., Dubois P. (2000). Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 28, 1–63. 10.1016/S0927-796X(00)00012-7 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

