Fermentative Production of l-2-Hydroxyglutarate by Engineered Corynebacterium glutamicum via Pathway Extension of l-Lysine Biosynthesis
- PMID: 33585425
- PMCID: PMC7873477
- DOI: 10.3389/fbioe.2020.630476
Fermentative Production of l-2-Hydroxyglutarate by Engineered Corynebacterium glutamicum via Pathway Extension of l-Lysine Biosynthesis
Abstract
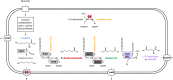
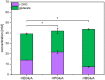
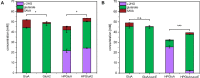
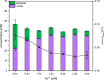
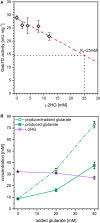
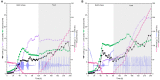
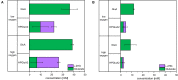
l-2-hydroxyglutarate (l-2HG) is a trifunctional building block and highly attractive for the chemical and pharmaceutical industries. The natural l-lysine biosynthesis pathway of the amino acid producer Corynebacterium glutamicum was extended for the fermentative production of l-2HG. Since l-2HG is not native to the metabolism of C. glutamicum metabolic engineering of a genome-streamlined l-lysine overproducing strain was required to enable the conversion of l-lysine to l-2HG in a six-step synthetic pathway. To this end, l-lysine decarboxylase was cascaded with two transamination reactions, two NAD(P)-dependent oxidation reactions and the terminal 2-oxoglutarate-dependent glutarate hydroxylase. Of three sources for glutarate hydroxylase the metalloenzyme CsiD from Pseudomonas putida supported l-2HG production to the highest titers. Genetic experiments suggested a role of succinate exporter SucE for export of l-2HG and improving expression of its gene by chromosomal exchange of its native promoter improved l-2HG production. The availability of Fe2+ as cofactor of CsiD was identified as a major bottleneck in the conversion of glutarate to l-2HG. As consequence of strain engineering and media adaptation product titers of 34 ± 0 mM were obtained in a microcultivation system. The glucose-based process was stable in 2 L bioreactor cultivations and a l-2HG titer of 3.5 g L-1 was obtained at the higher of two tested aeration levels. Production of l-2HG from a sidestream of the starch industry as renewable substrate was demonstrated. To the best of our knowledge, this study is the first description of fermentative production of l-2HG, a monomeric precursor used in electrochromic polyamides, to cross-link polyamides or to increase their biodegradability.
Keywords: C. glutamicum; L-2-hydroxyglutarate; bioreactor; glutarate hydroxylase; metabolic engineering; wheat sidestream concentrate.
Copyright © 2021 Prell, Burgardt, Meyer and Wendisch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Braun V. (1997). Avoidance of iron toxicity through regulation of bacterial iron transport. Biol. Chem. 378, 779–786. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

