Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects
- PMID: 33585430
- PMCID: PMC7873466
- DOI: 10.3389/fbioe.2021.603408
Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects
Abstract
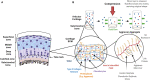
Articular cartilage is a highly specialised connective tissue of diarthrodial joints which provides a smooth, lubricated surface for joint articulation and plays a crucial role in the transmission of loads. In vivo cartilage is subjected to mechanical stimuli that are essential for cartilage development and the maintenance of a chondrocytic phenotype. Cartilage damage caused by traumatic injuries, ageing, or degradative diseases leads to impaired loading resistance and progressive degeneration of both the articular cartilage and the underlying subchondral bone. Since the tissue has limited self-repairing capacity due its avascular nature, restoration of its mechanical properties is still a major challenge. Tissue engineering techniques have the potential to heal osteochondral defects using a combination of stem cells, growth factors, and biomaterials that could produce a biomechanically functional tissue, representative of native hyaline cartilage. However, current clinical approaches fail to repair full-thickness defects that include the underlying subchondral bone. Moreover, when tested in vivo, current tissue-engineered grafts show limited capacity to regenerate the damaged tissue due to poor integration with host cartilage and the failure to retain structural integrity after insertion, resulting in reduced mechanical function. The aim of this review is to examine the optimal characteristics of osteochondral scaffolds. Additionally, an overview on the latest biomaterials potentially able to replicate the natural mechanical environment of articular cartilage and their role in maintaining mechanical cues to drive chondrogenesis will be detailed, as well as the overall mechanical performance of grafts engineered using different technologies.
Keywords: articular cartilage; biomaterials; mechanical testing; mechanobiology; osteochondral defects; stem cells; tissue engineering.
Copyright © 2021 Davis, Roldo, Blunn, Tozzi and Roncada.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

