Discovering the Potential of Dental Pulp Stem Cells for Corneal Endothelial Cell Production: A Proof of Concept
- PMID: 33585434
- PMCID: PMC7876244
- DOI: 10.3389/fbioe.2021.617724
Discovering the Potential of Dental Pulp Stem Cells for Corneal Endothelial Cell Production: A Proof of Concept
Abstract
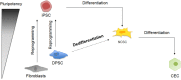
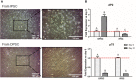
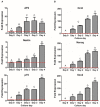
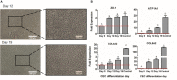
Failure of corneal endothelium cell monolayer is the main cause leading to corneal transplantation. Autologous cell-based therapies are required to reconstruct in vitro the cell monolayer. Several strategies have been proposed using embryonic stem cells and induced pluripotent stem cells, although their use has ethical issues as well as limited clinical applications. For this purpose, we propose the use of dental pulp stem cells isolated from the third molars to form the corneal endothelium cell monolayer. We hypothesize that using dental pulp stem cells that share an embryological origin with corneal endothelial cells, as they both arise from the neural crest, may allow a direct differentiation process avoiding the use of reprogramming techniques, such as induced pluripotent stem cells. In this work, we report a two-step differentiation protocol, where dental pulp stem cells are derived into neural crest stem-like cells and, then, into corneal endothelial-like cells. Initially, for the first-step we used an adhesion culture and compared two initial cell sources: a direct formation from dental pulp stem cells with the differentiation from induced pluripotent stem cells. Results showed significantly higher levels of early stage marker AP2 for the dental pulp stem cells compared to induced pluripotent stem cells. In order to provide a better environment for neural crest stem cells generation, we performed a suspension method, which induced the formation of neurospheres. Results showed that neurosphere formation obtained the peak of neural crest stem cell markers expression after 4 days, showing overexpression of AP2, Nestin, and p75 markers, confirming the formation of neural crest stem-like cells. Furthermore, pluripotent markers Oct4, Nanog, and Sox2 were as well-upregulated in suspension culture. Neurospheres were then directly cultured in corneal endothelial conditioned medium for the second differentiation into corneal endothelial-like cells. Results showed the conversion of dental pulp stem cells into polygonal-like cells expressing higher levels of ZO-1, ATP1A1, COL4A2, and COL8A2 markers, providing a proof of the conversion into corneal endothelial-like cells. Therefore, our findings demonstrate that patient-derived dental pulp stem cells may represent an autologous cell source for corneal endothelial therapies that avoids actual transplantation limitations as well as reprogramming techniques.
Keywords: cell reprogramming; cornea; corneal endothelium; dental pulp stem cells; differentiation; neural crest.
Copyright © 2021 Bosch, Salero, Núñez-Toldrà, Sabater, Gil and Perez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Therapeutic Potency of Induced Pluripotent Stem-Cell-Derived Corneal Endothelial-like Cells for Corneal Endothelial Dysfunction.Int J Mol Sci. 2022 Dec 31;24(1):701. doi: 10.3390/ijms24010701. Int J Mol Sci. 2022. PMID: 36614165 Free PMC article.
-
Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and Wnt/β-catenin signaling.Stem Cells Dev. 2013 Mar 1;22(5):828-39. doi: 10.1089/scd.2012.0286. Epub 2012 Oct 19. Stem Cells Dev. 2013. PMID: 22974347
-
Induction of neural crest cells from human dental pulp-derived induced pluripotent stem cells.Biomed Res. 2017;38(2):135-147. doi: 10.2220/biomedres.38.135. Biomed Res. 2017. PMID: 28442664
-
Corneal Endothelial-like Cells Derived from Induced Pluripotent Stem Cells for Cell Therapy.Int J Mol Sci. 2023 Aug 4;24(15):12433. doi: 10.3390/ijms241512433. Int J Mol Sci. 2023. PMID: 37569804 Free PMC article. Review.
-
Dental stem cells: a future asset of ocular cell therapy.Expert Rev Mol Med. 2015 Nov 10;17:e20. doi: 10.1017/erm.2015.16. Expert Rev Mol Med. 2015. PMID: 26553416 Review.
Cited by
-
Design of functional biomaterials as substrates for corneal endothelium tissue engineering.Regen Biomater. 2022 Jul 29;9:rbac052. doi: 10.1093/rb/rbac052. eCollection 2022. Regen Biomater. 2022. PMID: 35958516 Free PMC article. Review.
-
Biomedical Application of MSCs in Corneal Regeneration and Repair.Int J Mol Sci. 2025 Jan 15;26(2):695. doi: 10.3390/ijms26020695. Int J Mol Sci. 2025. PMID: 39859409 Free PMC article. Review.
-
Botanicals and Oral Stem Cell Mediated Regeneration: A Paradigm Shift from Artificial to Biological Replacement.Cells. 2022 Sep 7;11(18):2792. doi: 10.3390/cells11182792. Cells. 2022. PMID: 36139367 Free PMC article. Review.
-
DMP1-mediated transformation of DPSCs to CD31+/CD144+ cells demonstrate endothelial phenotype both in vitro and in vivo.Front Cell Dev Biol. 2025 Aug 8;13:1630129. doi: 10.3389/fcell.2025.1630129. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40861275 Free PMC article.
-
Recent Advances in Natural Materials for Corneal Tissue Engineering.Bioengineering (Basel). 2021 Oct 26;8(11):161. doi: 10.3390/bioengineering8110161. Bioengineering (Basel). 2021. PMID: 34821727 Free PMC article. Review.
References
-
- Baker M. (2008). Embryoid bodies get organized. Nat. Reports Stem Cells. 10.1038/stemcells.2008.146 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

