Promoter Methylation Leads to Decreased ZFP36 Expression and Deregulated NLRP3 Inflammasome Activation in Psoriatic Fibroblasts
- PMID: 33585499
- PMCID: PMC7874095
- DOI: 10.3389/fmed.2020.579383
Promoter Methylation Leads to Decreased ZFP36 Expression and Deregulated NLRP3 Inflammasome Activation in Psoriatic Fibroblasts
Abstract
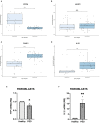
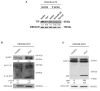
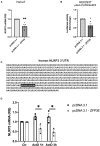
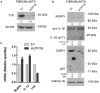
The mRNA-destabilizing protein tristetraprolin (TTP), encoded by the ZFP36 gene, is known to be able to end inflammatory responses by directly targeting and destabilizing mRNAs encoding pro-inflammatory cytokines. We analyzed its role in psoriasis, a disease characterized by chronic inflammation. We observed that TTP is downregulated in fibroblasts deriving from psoriasis patients compared to those deriving from healthy individuals and that psoriatic fibroblasts exhibit abnormal inflammasome activity compared to their physiological counterpart. This phenomenon depends on TTP downregulation. In fact, following restoration, TTP is capable of directly targeting for degradation NLRP3 mRNA, thereby drastically decreasing inflammasome activation. Moreover, we provide evidence that ZFP36 undergoes methylation in psoriasis, by virtue of the presence of long stretches of CpG dinucleotides both in the promoter and the coding region. Besides confirming that a perturbation of TTP expression might underlie the pathogenesis of psoriasis, we suggest that deregulated inflammasome activity might play a role in the disease alongside deregulated cytokine expression.
Keywords: NLRP3; ZFP36; inflammasome; methylation; psoriasis.
Copyright © 2021 Bertesi, Fantini, Alecci, Lotti, Martello, Parenti, Carretta, Marconi, Grande, Pincelli and Zanocco-Marani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

