MAC Project-Monitoring Anticoagulant Therapy Observational Study: Rationale and Protocol
- PMID: 33585500
- PMCID: PMC7876063
- DOI: 10.3389/fmed.2020.584459
MAC Project-Monitoring Anticoagulant Therapy Observational Study: Rationale and Protocol
Abstract
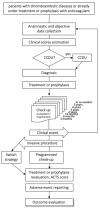
Real-life studies complement data from registrative trials. Because of the delayed registration of direct oral anticoagulants in Italy, scarce real-life data on such treatments is available for the Italian population. The aim of the MAC project is to collect real-life clinical information in unselected patients given oral anticoagulants for venous thromboembolism, during a 5-year follow-up period. This is a prospective-cohort, multi-center, observational study performed in four Italian centers. The estimated samples size is 4,000 patients. The efficacy outcomes are: incidence of symptomatic recurrent venous thromboembolism and of post-thrombotic syndrome. The safety outcomes are: incidence of major bleeding, clinically relevant non-major bleeding, minor bleeding, serious adverse events, and mortality. The MAC project has the potential to improve our understanding of the epidemiology and of the therapeutic strategies adopted in Italian patients with venous thromboembolism. Clinical Trial Registration: WWW.ClinicalTrials.Gov, identifier: NCT0432939.
Trial registration: ClinicalTrials.gov NCT00432939.
Keywords: anticoagulants; deep vein thrombosis; direct oral anticoagulant; monitoring; pulmonary embolism.
Copyright © 2021 Camporese, Bernardi, Bortoluzzi, Noventa, Hong, Callegari, Villalta, Tonello, Nardin, Campello, Spiezia and Simioni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. European Heart J. (2017) 38:2137–49. 10.1093/eurheartj/ehw058 - DOI - PMC - PubMed
-
- Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth R, et al. Stroke, bleeding, and mortality risks in elderly medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. (2016) 176:1662–71. 10.1001/jamainternmed.2016.5954 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical