Wildlife as Sentinels of Antimicrobial Resistance in Germany?
- PMID: 33585611
- PMCID: PMC7873465
- DOI: 10.3389/fvets.2020.627821
Wildlife as Sentinels of Antimicrobial Resistance in Germany?
Abstract
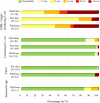
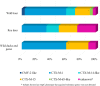
The presence of bacteria carrying antimicrobial resistance (AMR) genes in wildlife is an indicator that resistant bacteria of human or livestock origin are widespread in the environment. In addition, it could represent an additional challenge for human health, since wild animals could act as efficient AMR reservoirs and epidemiological links between human, livestock and natural environments. The aim of this study was to investigate the occurrence and the antibiotic resistance patterns of several bacterial species in certain wild animals in Germany, including wild boars (Sus scrofa), roe deer (Capreolus capreolus) and wild ducks (family Anatidae, subfamily Anatinae) and geese (family Anatidae, subfamily Anserinae). In the framework of the German National Zoonoses Monitoring Program, samples from hunted wild boars, roe deer and wild ducks and geese were collected nationwide in 2016, 2017, and 2019, respectively. Fecal samples were tested for the presence of Salmonella spp. (in wild boars and wild ducks and geese), Campylobacter spp. (in roe deer and wild ducks and geese), Shiga toxin-producing Escherichia (E.) coli (STEC), commensal E. coli and extended-spectrum beta-lactamase- (ESBL) or ampicillinase class C (AmpC) beta-lactamase-producing E. coli (in wild boars, roe deer and wild ducks and geese). In addition, the presence of methicillin-resistant Staphylococcus aureus (MRSA) was investigated in nasal swabs from wild boars. Isolates obtained in the accredited regional state laboratories were submitted to the National Reference Laboratories (NRLs) for confirmation, characterization and phenotypic resistance testing using broth microdilution according to CLSI. AMR was assessed according to epidemiological cut-offs provided by EUCAST. Salmonella spp. were isolated from 13 of 552 (2.4%) tested wild boar fecal samples, but absent in all 101 samples from wild ducks and geese. Nine of the 11 isolates that were submitted to the NRL Salmonella were susceptible to all tested antimicrobial substances. Campylobacter spp. were isolated from four out of 504 (0.8%) roe deer fecal samples, but not from any of the samples from wild ducks and geese. Of the two isolates received in the NRL Campylobacter, neither showed resistance to any of the substances tested. From roe deer, 40.2% of the fecal samples (144 of 358) yielded STEC compared to 6.9% (37 of 536) from wild boars. In wild ducks and geese, no STEC isolates were found. Of 150 STEC isolates received in the NRL (24 from wild boars and 126 from roe deer), only one from each animal species showed resistance. Of the 219 isolates of commensal E. coli from wild boars tested for AMR, 210 were susceptible to all 14 tested substances (95.9%). In roe deer this proportion was even higher (263 of 269, 97.8%), whereas in wild ducks and geese this proportion was lower (41 of 49, 83.7%). Nevertheless, selective isolation of ESBL-/AmpC-producing E. coli yielded 6.5% (36 of 551) positive samples from wild boars, 2.3% (13 of 573) from roe deer and 9.8% (10 of 102) from wild ducks and geese. Among the 25 confirmed ESBL-/AmpC-producing isolates from wild boars, 14 (56.0%) showed resistance up to five classes of substances. This proportion was lower in roe deer (3 of 12, 25%) and higher in wild ducks and geese (7 of 10, 70%). None of the 577 nasal swabs from wild boars yielded MRSA. Results indicate that overall, the prevalence of resistant bacteria from certain wild animals in Germany is low, which may reflect not only the low level of exposure to antimicrobials but also the low level of resistant bacteria in the areas where these animals live and feed. However, despite this low prevalence, the patterns observed in bacteria from the wild animals included in this study are an indicator for specific resistance traits in the environment, including those to highest priority substances such as 3rd generation cephalosporins, fluoroquinolones and colistin. Therefore, also continuous monitoring of the occurrence of such bacteria in wildlife by selective isolation is advisable. Furthermore, the possible role of wildlife as reservoir and disperser of resistant bacteria would need to be assessed, as wild animals, and in particular wild ducks and geese could become spreaders of resistant bacteria given their capacity for long-range movements.
Keywords: Germany; antimicrobial resistance (AMR); cervids; monitoring; one health; wild bird; wild boar; zoonotic agents.
Copyright © 2021 Plaza-Rodríguez, Alt, Grobbel, Hammerl, Irrgang, Szabo, Stingl, Schuh, Wiehle, Pfefferkorn, Naumann, Kaesbohrer and Tenhagen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- WHO (2014). Antimicrobial Resistance: Global Report on Surveillance. Jeneva: World Health Organization.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

