Outcomes with a shorter multidrug-resistant tuberculosis regimen from Karakalpakstan, Uzbekistan
- PMID: 33585652
- PMCID: PMC7869592
- DOI: 10.1183/23120541.00537-2020
Outcomes with a shorter multidrug-resistant tuberculosis regimen from Karakalpakstan, Uzbekistan
Abstract
Background: In 2016, World Health Organization guidelines conditionally recommended standardised shorter 9-12-month regimens for multidrug-resistant (MDR) tuberculosis (TB) treatment. We conducted a prospective study of a shorter standardised MDR-TB regimen in Karakalpakstan, Uzbekistan.
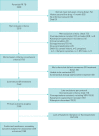
Methods: Consecutive adults and children with confirmed rifampicin-resistant pulmonary TB were enrolled between September 1, 2013 and March 31, 2015; exclusions included prior treatment with second-line anti-TB drugs, and documented resistance to ofloxacin or to two second-line injectable agents. The primary outcome was recurrence-free cure at 1 year following treatment completion.
Results: Of 146 enrolled patients, 128 were included: 67 female (52.3%), median age 30.1 (interquartile range 23.8-44.4) years. At the end of treatment, 71.9% (92 out of 128) of patients achieved treatment success, with 68% (87 out of 128) achieving recurrence-free cure at 1 year following completion. Unsuccessful outcomes during treatment included 22 (17.2%) treatment failures with fluoroquinolone-resistance amplification in 8 patients (8 out of 22, 36.4%); 12 (9.4%) lost to follow-up; and 2 (1.5%) deaths. Recurrence occurred in one patient. Fourteen patients (10.9%) experienced serious adverse events. Baseline resistance to both pyrazinamide and ethambutol (adjusted OR 6.13, 95% CI 2.01; 18.63) and adherence <95% (adjusted OR 5.33, 95% CI 1.73; 16.36) were associated with unsuccessful outcome in multivariable logistic regression.
Conclusions: Overall success with a standardised shorter MDR-TB regimen was moderate with considerable treatment failure and amplification of fluoroquinolone resistance. When introducing standardised shorter regimens, baseline drug susceptibility testing and minimising missed doses are critical. High rates globally of pyrazinamide, ethambutol and ethionamide resistance raise questions of continued inclusion of these drugs in shorter regimens in the absence of drug susceptibility testing-confirmed susceptibility.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: P. du Cros reports other funded work from TB Alliance for introduction of pretomanid, outside the submitted work. Conflict of interest: A. Khamraev has nothing to disclose. Conflict of interest: Z. Tigay has nothing to disclose. Conflict of interest: T. Abdrasuliev has nothing to disclose. Conflict of interest: J. Greig has nothing to disclose. Conflict of interest: G. Cooke has nothing to disclose. Conflict of interest: K. Herboczek has nothing to disclose. Conflict of interest: T. Pylypenko has nothing to disclose. Conflict of interest: C. Berry has nothing to disclose. Conflict of interest: A. Ronnachit has nothing to disclose. Conflict of interest: D. Lister has nothing to disclose. Conflict of interest: S. Dietrich has nothing to disclose. Conflict of interest: C. Ariti has nothing to disclose. Conflict of interest: K. Safaev has nothing to disclose. Conflict of interest: B-T. Nyang'wa has nothing to disclose. Conflict of interest: N. Parpieva has nothing to disclose. Conflict of interest: M. Tillashaykhov has nothing to disclose. Conflict of interest: J. Achar has nothing to disclose.
Figures
References
-
- World Health Organization Global Tuberculosis Report 2019. Geneva, World Health Organization, 2019.
LinkOut - more resources
Full Text Sources