Rates of gene conversions between Escherichia coli ribosomal operons
- PMID: 33585862
- PMCID: PMC8022953
- DOI: 10.1093/g3journal/jkaa002
Rates of gene conversions between Escherichia coli ribosomal operons
Abstract
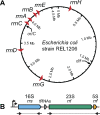
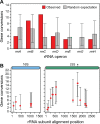
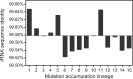
Due to their universal presence and high sequence conservation, ribosomal RNA (rRNA) sequences are used widely in phylogenetics for inferring evolutionary relationships between microbes and in metagenomics for analyzing the composition of microbial communities. Most microbial genomes encode multiple copies of rRNA genes to supply cells with sufficient capacity for protein synthesis. These copies typically undergo concerted evolution that keeps their sequences identical, or nearly so, due to gene conversion, a type of intragenomic recombination that changes one copy of a homologous sequence to exactly match another. Widely varying rates of rRNA gene conversion have previously been estimated by comparative genomics methods and using genetic reporter assays. To more directly measure rates of rRNA intragenomic recombination, we sequenced the seven Escherichia coli rRNA operons in 15 lineages that were evolved for ∼13,750 generations with frequent single-cell bottlenecks that reduce the effects of selection. We identified 38 gene conversion events and estimated an overall rate of intragenomic recombination within the 16S and 23S genes between rRNA copies of 3.6 × 10-4 per genome per generation or 8.6 × 10-6 per rRNA operon per homologous donor operon per generation. This rate varied only slightly from random expectations at different sites within the rRNA genes and between rRNA operons located at different positions in the genome. Our accurate estimate of the rate of rRNA gene conversions fills a gap in our quantitative understanding of how ribosomal sequences and other multicopy elements diversify and homogenize during microbial genome evolution.
Keywords: 16S sequencing; concerted evolution; gene conversion; mutation accumulation experiment; mutation rate; ribosomal RNA.
© The Author(s) 2020. Published by Oxford University Press on behalf of Genetics Society of America.
Figures


References
-
- Abdulkarim F, Hughes D.. 1996. Homologous recombination between the tuf genes of Salmonella typhimurium. J Mol Biol. 260:506–522. - PubMed
-
- Ceroni F, Algar R, Stan G-B, Ellis T.. 2015. Quantifying cellular capacity identifies gene expression designs with reduced burden. Nat Methods. 12:415–418. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous
