Whole-genome assembly of Culex tarsalis
- PMID: 33585869
- PMCID: PMC8022977
- DOI: 10.1093/g3journal/jkaa063
Whole-genome assembly of Culex tarsalis
Abstract
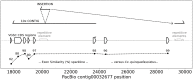
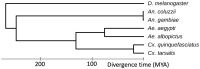
The mosquito, Culex tarsalis, is a key vector in the western United States due to its role in transmission of zoonotic arboviruses that affect human health. Extensive research has been conducted on Cx. tarsalis ecology, feeding behavior, vector competence, autogeny, diapause, genetics, and insecticide resistance. Population genetic analyses in the western U.S. have identified at least three genetic clusters that are geographically distinct. However, in-depth genetic studies have been hindered by the lack of a reference genome. In this study, we present the first whole-genome assembly of this mosquito species (CtarK1) based on PacBio HiFi reads from high-molecular-weight DNA extracted from a single male. The CtarK1 assembly is 790 Mb with an N50 of 58 kb, which is 27% larger than Culex quinquefasciatus (578 Mb). This difference appears to be mostly composed of transposable elements. To annotate CtarK1, we used a previously assembled Cx. tarsalis transcriptome and approximately 17,456 protein genes from Cx. quinquefasciatus (N = 17,456). Genome completeness was assessed using the Benchmarking Universal Single-Copy Orthologs (BUSCO) tool, which identified 84.8% of the 2799 Dipteran BUSCO genes. Using a Bayesian phylogeny based on mitochondrial genomes, we place Cx. tarsalis in the context of other mosquito species and estimate the divergence between Cx. tarsalis and Cx. quinquefasciatus to be between 15.8 and 22.2 million years ago (MYA). Important next steps from this work include characterizing the genetic basis of diapause and sex determination in Culex mosquitoes.
Keywords: Genetics; Genome; PacBio; Vector; mosquito.
© The Author(s) 2021. Published by Oxford University Press on behalf of Genetics Society of America.
Figures

References
-
- Buth JL, Brust RA, Ellis RA.. 1990. Development time, oviposition activity and onset of diapause in Culex tarsalis, Culex restuans and Culiseta inornata in southern Manitoba. J Am Mosq Control Assoc. 6:55–63. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
