Mechanisms of neuronal dysfunction in HIV-associated neurocognitive disorders
- PMID: 33585975
- PMCID: PMC8164580
- DOI: 10.1007/s00018-021-03785-y
Mechanisms of neuronal dysfunction in HIV-associated neurocognitive disorders
Abstract
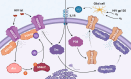
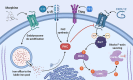
HIV-associated neurocognitive disorder (HAND) is characterized by cognitive and behavioral deficits in people living with HIV. HAND is still common in patients that take antiretroviral therapies, although they tend to present with less severe symptoms. The continued prevalence of HAND in treated patients is a major therapeutic challenge, as even minor cognitive impairment decreases patient's quality of life. Therefore, modern HAND research aims to broaden our understanding of the mechanisms that drive cognitive impairment in people with HIV and identify promising molecular pathways and targets that could be exploited therapeutically. Recent studies suggest that HAND in treated patients is at least partially induced by subtle synaptodendritic damage and disruption of neuronal networks in brain areas that mediate learning, memory, and executive functions. Although the causes of subtle neuronal dysfunction are varied, reversing synaptodendritic damage in animal models restores cognitive function and thus highlights a promising therapeutic approach. In this review, we examine evidence of synaptodendritic damage and disrupted neuronal connectivity in HAND from clinical neuroimaging and neuropathology studies and discuss studies in HAND models that define structural and functional impairment of neurotransmission. Then, we report molecular pathways, mechanisms, and comorbidities involved in this neuronal dysfunction, discuss new approaches to reverse neuronal damage, and highlight current gaps in knowledge. Continued research on the manifestation and mechanisms of synaptic injury and network dysfunction in HAND patients and experimental models will be critical if we are to develop safe and effective therapies that reverse subtle neuropathology and cognitive impairment.
Keywords: Cognitive impairment; Dendritic spines; Drug abuse; HAND; Neuroinflammation; Neuronal connectivity.
Figures

References
-
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. - DOI - PMC - PubMed
-
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8(2):136–142. doi: 10.1080/13550280290049615. - DOI - PubMed
-
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

