Plasma p-tau231: a new biomarker for incipient Alzheimer's disease pathology
- PMID: 33585983
- PMCID: PMC8043944
- DOI: 10.1007/s00401-021-02275-6
Plasma p-tau231: a new biomarker for incipient Alzheimer's disease pathology
Abstract
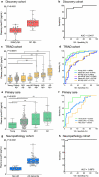
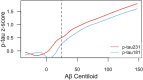
The quantification of phosphorylated tau in biofluids, either cerebrospinal fluid (CSF) or plasma, has shown great promise in detecting Alzheimer's disease (AD) pathophysiology. Tau phosphorylated at threonine 231 (p-tau231) is one such biomarker in CSF but its usefulness as a blood biomarker is currently unknown. Here, we developed an ultrasensitive Single molecule array (Simoa) for the quantification of plasma p-tau231 which was validated in four independent cohorts (n = 588) in different settings, including the full AD continuum and non-AD neurodegenerative disorders. Plasma p-tau231 was able to identify patients with AD and differentiate them from amyloid-β negative cognitively unimpaired (CU) older adults with high accuracy (AUC = 0.92-0.94). Plasma p-tau231 also distinguished AD patients from patients with non-AD neurodegenerative disorders (AUC = 0.93), as well as from amyloid-β negative MCI patients (AUC = 0.89). In a neuropathology cohort, plasma p-tau231 in samples taken on avergae 4.2 years prior to post-mortem very accurately identified AD neuropathology in comparison to non-AD neurodegenerative disorders (AUC = 0.99), this is despite all patients being given an AD dementia diagnosis during life. Plasma p-tau231 was highly correlated with CSF p-tau231, tau pathology as assessed by [18F]MK-6240 positron emission tomography (PET), and brain amyloidosis by [18F]AZD469 PET. Remarkably, the inflection point of plasma p-tau231, increasing as a function of continuous [18F]AZD469 amyloid-β PET standardized uptake value ratio, was shown to be earlier than standard thresholds of amyloid-β PET positivity and the increase of plasma p-tau181. Furthermore, plasma p-tau231 was significantly increased in amyloid-β PET quartiles 2-4, whereas CSF p-tau217 and plasma p-tau181 increased only at quartiles 3-4 and 4, respectively. Finally, plasma p-tau231 differentiated individuals across the entire Braak stage spectrum, including Braak staging from Braak 0 through Braak I-II, which was not observed for plasma p-tau181. To conclude, this novel plasma p-tau231 assay identifies the clinical stages of AD and neuropathology equally well as plasma p-tau181, but increases earlier, already with subtle amyloid-β deposition, prior to the threshold for amyloid-β PET positivity has been attained, and also in response to early brain tau deposition. Thus, plasma p-tau231 is a promising novel biomarker of emerging AD pathology with the potential to facilitate clinical trials to identify vulnerable populations below PET threshold of amyloid-β positivity or apparent entorhinal tau deposition.
Keywords: Alzheimer’s disease; Biomarkers; Blood; Braak; Preclinical; Tau; p-tau181; p-tau217; p-tau231.
Conflict of interest statement
HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, and has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen. KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. EVM is a co-founder of ADx NeuroSciences. The other authors declare no competing interest.
Figures



References
-
- Ashton NJ, Benedet AL, Pascoal TA, Karikari TK, Lantero-Rodriguez J, Mathotaarachchi S, Therriault J, Savard M, Chamoun M, Stoops E et al (2021) Cerebrospinal fluid p-tau231 as an early indicator of emerging pathology in Alzheimer’s disease. Research Square. 10.21203/rs.3.rs-155736/v1 (PREPRINT (Version 1)) - PMC - PubMed
-
- Ashton NJ, Leuzy A, Lim YM, Troakes C, Hortobagyi T, Hoglund K, Aarsland D, Lovestone S, Scholl M, Blennow K, et al. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Commun. 2019;7:5. doi: 10.1186/s40478-018-0649-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

