Aldosterone antagonists for people with chronic kidney disease requiring dialysis
- PMID: 33586138
- PMCID: PMC8094170
- DOI: 10.1002/14651858.CD013109.pub2
Aldosterone antagonists for people with chronic kidney disease requiring dialysis
Abstract
Background: People with chronic kidney disease (CKD) requiring dialysis are at a particularly high risk of cardiovascular death and morbidity. Several clinical studies suggested that aldosterone antagonists would be a promising treatment option for people undergoing dialysis. However, the clinical efficacy and potential harm of aldosterone antagonists for people with CKD on dialysis has yet to be determined.
Objectives: This review aimed to evaluate the benefits and harms of aldosterone antagonists, both non-selective (spironolactone) and selective (eplerenone), in comparison to control (placebo or standard care) in people with CKD requiring haemodialysis (HD) or peritoneal dialysis (PD).
Search methods: We searched the Cochrane Kidney and Transplant Register of Studies up to 5 August 2020 using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: We included parallel randomised controlled trials (RCTs), cross-over RCTs, and quasi-RCTs (where group allocation is by a method that is not truly random, such as alternation, assignment based on alternate medical records, date of birth, case record number, or other predictable methods) that compared aldosterone antagonists with placebo or standard care in people with CKD requiring dialysis.
Data collection and analysis: Two review authors independently extracted data and assessed risk of bias for included studies. We used a random-effects model meta-analysis to perform a quantitative synthesis of the data. We used the I² statistic to measure heterogeneity among the studies in each analysis. We indicated summary estimates as a risk ratio (RR) for dichotomous outcomes, mean difference (MD) for continuous outcomes, or standardised mean differences (SMD) if different scales were used, with their 95% confidence interval (CI). We assessed the certainty of the evidence for each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) approach.
Main results: We included 16 studies (14 parallel RCTs and two cross-over RCTs) involving a total of 1446 participants. Thirteen studies compared spironolactone to placebo or standard care and one study compared eplerenone to a placebo. Most included studies had an unclear or high risk of bias. Compared to control, aldosterone antagonists probably reduced the risk of death (any cause) for people with CKD requiring dialysis (9 studies, 1119 participants: RR 0.45, 95% CI 0.30 to 0.67; I² = 0%; moderate certainty of evidence). Aldosterone antagonist probably decreased the risk of death due to cardiovascular disease (6 studies, 908 participants: RR 0.37, 95% CI 0.22 to 0.64; I² = 0%; moderate certainty of evidence) and cardiovascular and cerebrovascular morbidity (3 studies, 328 participants: RR 0.38, 95% CI 0.18 to 0.76; I² = 0%; moderate certainty of evidence). While aldosterone antagonists probably increased risk of gynaecomastia compared with control (4 studies, 768 participants: RR 5.95, 95% CI 1.93 to 18.3; I² = 0%; moderate certainty of evidence), aldosterone antagonists may make little or no difference to the risk of hyperkalaemia (9 studies, 981 participants: RR 1.41, 95% CI 0.72 to 2.78; I² = 47%; low certainty of evidence). Aldosterone antagonists had a marginal effect on left ventricular mass among participants undergoing dialysis (8 studies, 633 participants: SMD -0.42, 95% CI -0.78 to 0.05; I² = 77%). In people with CKD requiring dialysis received aldosterone antagonists compared to control, there were 72 fewer deaths from all causes per 1000 participants (95% CI 47 to 98) with a number needed to treat for an additional beneficial outcome (NNTB) of 14 (95% CI 10 to 21) and for gynaecomastia were 26 events per 1000 participants (95% CI 15 to 39) with a number need to treat for an additional harmful outcome (NNTH) of 38 (95% CI 26 to 68).
Authors' conclusions: Based on moderate certainty of the evidence, aldosterone antagonists probably reduces the risk of all-cause and cardiovascular death and probably reduces morbidity due to cardiovascular and cerebrovascular disease in people with CKD requiring dialysis. For the adverse effect of gynaecomastia, the risk was increased compared to control. For this outcome, the absolute risk was lower than the absolute risk of death. It is hoped the three large ongoing studies will provide better certainty of evidence.
Trial registration: ClinicalTrials.gov NCT01128101 NCT01687699 NCT01691053 NCT01650012 NCT02285920 NCT00865449 NCT02490904 NCT01855334 NCT00328809 NCT03314493 NCT02190318 NCT03953950 NCT03020303 NCT01848639 NCT00277693.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
TH: has a grant by JSPS KAKENHI Grant Number 19K03092 and consultancy agreement with Kyowa Kirin and has received speaker honoraria from Kyowa Kirin, Astellas, Baxter, Terumo, and Torii Pharmaceutical for activities unrelated to this review
H Nishiwaki: has declared that they have no conflict of interest
EO: has declared that they have no conflict of interest
WL: has declared that they have no conflict of interest
H Noma: has received consultant fees from Kyowa Kirinand lecture fees from Boehringer Ingelheim and Kyowa Kirin
Figures


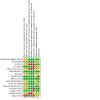












Update of
References
References to studies included in this review
Feniman De Stefano 2015 {published data only}
-
- Feniman-De-Stefano GM, Zanati-Basan SG, De Stefano LM, Xavier PS, Castro AD, Caramori JC, et al. Spironolactone is secure and reduces left ventricular hypertrophy in hemodialysis patients. Therapeutic Advances in Cardiovascular Disease 2015;9(4):158-67. [MEDLINE: ] - PubMed
Gross 2005 {published data only}
-
- Gross E, Rothstein M, Dombek S, Juknis HI. Effect of spironolactone on blood pressure and the renin-angiotensin-aldosterone system in oligo-anuric hemodialysis patients. American Journal of Kidney Diseases 2005;46(1):94-101. [MEDLINE: ] - PubMed
Ito 2014 {published data only}
-
- Ito Y, Mizuno M, Suzuki Y, Kasuga H, Maruyama S, Yuzawa Y, et al. Spironolactone prevents cardiac hypertrophy in peritoneal dialysis patients without significant adverse effects [abstract no: SA-OR127]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):100A.
Lin 2016a {published data only}
Matsumoto 2014 {published data only}
-
- Matsumoto Y, Kageyama S, Mori Y, Yakushigawa T, Arihara K. Spironolactone reduces cardio-and cerebrovascular morbidity and mortality in hemodialysis patients [abstract no: FR-OR025]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):36A. - PubMed
-
- Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. Journal of the American College of Cardiology 2014;63(6):528-36. [MEDLINE: ] - PubMed
MiREnDa 2014 {published data only}
-
- Hammer F, Krane V, Stork S, Roser C, Hofmann K, Pollak N, et al. Rationale and design of the Mineralocorticoid Receptor Antagonists in End-Stage Renal Disease Study (MiREnDa). Nephrology Dialysis Transplantation 2014;29(2):400-5. [MEDLINE: ] - PubMed
-
- Hammer F, Malzahn U, Donhauser J, Betz C, Schneider MP, Grupp C, et al. A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney International 2019;95(4):983-91. [MEDLINE: ] - PubMed
-
- Hauser T, Malzahn U, Betz C, Grupp C, Doeltz T, Grebe S, et al. The prognostic value of nephro-cardiac biomarkers in hemodialysis patients-the randomized-controlled MiREnDa trial [abstract no: FP532]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i218. [EMBASE: 622606301]
-
- Wanner C, Malzahn U, Donhauuser J, Betz C, Grupp C, Doltz T, et al. Effect of spironolactone on the left ventricular mass in hemodialysis patients: the MiREnDa study- a randomized controlled trial. [abstract no: SA-PO849]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):899.
Ni 2014 {published data only}
PHASE 2015 {published data only}
SPIN‐D 2019 {published data only}
-
- Charytan D, Himmelfarb J, Ikizler TA, Raj DS, Hsu JY, Landis JR, et al. Safety and cardiovascular efficacy of spironolactone (SPL) in dialysis-dependent ESRD (SPin-D): a pilot trial of the NIDDK Hemodialysis Novel Therapies Consortium [abstract no: FR-PO1078]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):B9.
Taheri CAPD 2012 {published data only}
-
- Najafabadi MM, Taheri S, Seyrafian S, Alipoor Z, Karimi S, Poormodhadas A, et al. Spironolactone in continuous ambulatory peritoneal dialysis patients, improves cardiac function [abstract no: SU475]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
-
- Taheri S, Mortazavi M, Pourmoghadas A, Seyrafian S, Alipour Z, Karimi S. A prospective double-blind randomized placebo-controlled clinical trial to evaluate the safety and efficacy of spironolactone in patients with advanced congestive heart failure on continuous ambulatory peritoneal dialysis. Saudi Journal of Kidney Diseases & Transplantation 2012;23(3):507-12. [MEDLINE: ] - PubMed
Taheri HD 2009 {published data only}
-
- Mortazavi M, Taheri S, Shahidi S, Pourmoghaddas A, Yaraghi MG, Seyrafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function [abstract no: FP379]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi145. [CENTRAL: CN-00716090] - PubMed
-
- Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi Journal of Kidney Diseases & Transplantation 2009;20(3):392-7. [MEDLINE: ] - PubMed
-
- Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function [abstract no: S-PO-0370]. In: 4th World Congress of Nephrology.19th International Congress of the International Society of Nephrology (ISN); 2007 Apr 21-25; Rio de Janeiro, Brazil. 2007:148.
Vazquez‐Rangel 2014 {published data only}
-
- Vazquez-Rangel A, Soto V, Escalona M, Toledo RG, Castillo EA, Polanco Flores NA, et al. Spironolactone to prevent peritoneal fibrosis in peritoneal dialysis patients: a randomized controlled trial. American Journal of Kidney Diseases 2014;63(6):1072-4. [MEDLINE: ] - PubMed
-
- Vazquez-Rangel A, Soto V, Toledo RG, Castillo EA, Polanco Flores NA, Falcon-Chavez I, et al. Spironolactone to prevent peritoneal fibrosis in peritoneal dialysis patients: a randomized controlled trial [abstract no: FR-PO789]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):549A. - PubMed
Vukusich 2010 {published data only}
-
- Vukusich A, Kunstmann S, Varela C, Gainza D, Bravo S, Sepulveda D, et al. A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(8):1380-7. [MEDLINE: ] - PMC - PubMed
Yongsiri 2015 {published data only}
-
- Yongsiri S, Thammakumpee J, Prongnamchai S, Tengpraettanakorn P, Chueansuwan R, Tangjaturonrasme S, et al. Randomized, double-blind, placebo-controlled trial of spironolactone for hypokalemia in continuous ambulatory peritoneal dialysis patients. Therapeutic Apheresis & Dialysis 2015;19(1):81-6. [MEDLINE: ] - PubMed
-
- Yongsiri S, Thammakumpee J, Prongnamchai S, Tengpraettanakorn P, Tangjaturonrasme S, Dinchuthai P, et al. Spironolactone for hypokalia in CAPD patients, a randomized, double-blind, placebo controlled trial [abstract no: 390]. American Journal of Kidney Diseases 2014;63(5):A116. [EMBASE: 71448657]
Zaripova 2012 {published data only}
-
- Zaripova I, Kayukov I, Essaian A, Nimgirova A. Renin angiotensin aldosterone system blockade and left ventricular hypertrophy in maintenance hemodialysis patients [abstract no: FP497]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii238. [EMBASE: 70765993]
Ziaee 2019 {published data only}
-
- Ziaee SA, Karvandi M, Ziaee NS, Ghozloujeh ZG, Shahrbaf MA, Roshan A. Effects of spironolactone on cardiovascular complications in hemodialysis patients of Taleghani Hospital during the period of 2016-2017: a randomized double-blind controlled clinical trial. Iranian Heart Journal 2019;20(1):45-52. [EMBASE: 2001576782]
References to studies excluded from this review
Eklund 2016 {published data only}
-
- Eklund M, Furuland H, Hellberg O, Nilsson E. Effect of spironolactone on vascular stiffness in hemodialysis - a randomized crossover study [abstract no: TH-PO913]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):305-6A.
EPURE 2018 {published data only}
Essaian 2007 {published data only}
-
- Essaian A, Kaukov I, Karabayeva A, Kadinskaya M, Katysheva N. The influence of spironolactone on plasma level of plasminogen-activator inhibitor type 1 (PAI-1) in anuric hemodialysis patients [abstract no: SaP266]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi322. [CENTRAL: CN-00716089]
Host 2000 {published data only}
-
- Host U, Egfjord M, Holm E, Jespersen B, Lokkegaard H, Skagen K, et al. Long term spironolactone and cardiac function in chronic uremia [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A215. [CENTRAL: CN-00460961]
Koh 2010 {published data only}
-
- Koh KH, Lee J, Chai NS, Tan C. Randomized controlled trial on the antihypertensive effect of spironolactone in addition to salt restriction in peritoneal dialysis patients [abstract]. Peritoneal Dialysis International 2010;30(Suppl 2):S137.
LAST‐D 2013 {published data only}
-
- Charytan D. L-arginine and spironolactone trial in dialysis-dependent ESRD (LAST-D). www.clinicaltrials.gov/ct2/show/NCT01855334 (first received 16 May 2013).
Nakao 2007 {published data only}
-
- Nakao N, Hasegawa H, Fujimori A, Seno H, Toriyama T, Kawahara H. Effects of combined b-blocker and anti-aldosterone antagonist treatment for cardiovascular prevention in patients receiving maintenance hemodialysis [abstract no: SU-PO573]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):709A.
NCT00328809 {published data only}
-
- Narsipur SS. Spironolactone safety in dialysis patients. www.clinicaltrials.gov/ct2/show/NCT00328809 (first received 22 May 2006).
References to studies awaiting assessment
Gueiros 2019 {published data only}
NCT02190318 {published data only}
-
- Liu H. Residual renal function preservation in peritoneal dialysis patients. www.clinicaltrials.gov/ct2/show/NCT02190318 (first received 15 July 2014).
NCT03953950 {published data only}
-
- Ruengorn C. Effect of add-on spironolactone to losartan versus losartan alone on peritoneal membrane among peritoneal dialysis patients (ESCAPE-PD). www.clinicaltrials.gov/show/nct03953950 (received 17 May 2019).
Song 2017 {published data only}
-
- Song, et al. Effect of spironolactone on cardiac function, dialysis adequacy and complications in hemodialysis. Journal of Modern Medicine & Health 2017;33(19):2947-9.
Wang 2018 {published data only}
-
- Wang CC, et al. Effects of spironolacone on cardiac and residual renal function in patients with peritoneal dialysis. Chinese Journal of General Practice 2018;16(8):1303-7.
References to ongoing studies
ACHIEVE 2017 {published data only}
-
- Walsh M, Devereaux PJ. Aldosterone bloCkade for Health Improvement EValuation in End-stage Renal Disease (ACHIEVE). www.clinicaltrials.gov/ct2/show/NCT03020303 (first received 13 January 2017).
ALCHEMIST 2014 {published data only}
-
- Rossignol P. ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial (ALCHEMIST). www.clinicaltrials.gov/ct2/show/NCT01848639 (first received 7 May 2013).
NCT00277693 {published data only}
-
- Michea LF. Cardiovascular protective effect of spironolactone in hemodialysis. www.clinicaltrials.gov/ct2/show/NCT00277693 (first received 16 January 2006).
Additional references
Bolignano 2014
Feniman‐De‐Stefano 2015
-
- Feniman-De-Stefano GM, Zanati-Basan SG, De Stefano LM, Xavier PS, Castro AD, Caramori JC, et al. Spironolactone is secure and reduces left ventricular hypertrophy in hemodialysis patients. Therapeutic Advances in Cardiovascular Disease 2015;9(4):158-67. [MEDLINE: ] - PubMed
Foley 1995
-
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney International 1995;47(1):186-92. [MEDLINE: ] - PubMed
Foley 1998
-
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. American Journal of Kidney Diseases 1998;32(5 Suppl 3):S112-9. [MEDLINE: ] - PubMed
GRADE 2008
GRADE 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] - PubMed
Guyatt 2011
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence-imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [MEDLINE: ] - PubMed
Harnett 1995
-
- Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients: Prevalence, incidence, prognosis and risk factors. Kidney International 1995;47(3):884-90. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Levey 2011
-
- Levey AS, Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report.[Erratum appears in Kidney Int. 2011 Nov;80(9):1000], [Erratum appears in Kidney Int. 2011 Nov 1;80(9):1000; PMID: 30036909]. Kidney International 2011;80(1):17-28. [MEDLINE: ] - PubMed
Li 2019
Liyanage 2015
-
- Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015;385(9981):1975-82. [MEDLINE: ] - PubMed
Pitt 1999
-
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. New England Journal of Medicine 1999;341(10):709-17. [MEDLINE: ] - PubMed
Pitt 2003
-
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction.[Erratum in: N Engl J Med. 2003 May 29;348(22):2271]. New England Journal of Medicine 2003;348(14):1309-21. [MEDLINE: ] - PubMed
Quach 2016
-
- Quach K, Lvtvyn L, Baigent C, Bueti J, Garg AX, Hawley C, et al. The safety and efficacy of mineralocorticoid receptor antagonists in patients who require dialysis: a systematic review and meta-analysis. American Journal of Kidney Diseases 2016;68(4):591-8. [MEDLINE: ] - PubMed
Salem 1995
-
- Salem MM. Hypertension in the hemodialysis population: a survey of 649 patients. American Journal of Kidney Diseases 1995;26(3):461-8. [MEDLINE: ] - PubMed
Sato 1999
-
- Sato A, Funder JW, Saruta T. Involvement of aldosterone in left ventricular hypertrophy of patients with end-stage renal failure treated with hemodialysis. American Journal of Hypertension 1999;12(9 Pt 1):867-73. [MEDLINE: ] - PubMed
Saudan 2003
-
- Saudan P, Mach F, Perneger T, Schnetzler B, Stoermann C, Fumeaux Z, et al. Safety of low-dose spironolactone administration in chronic haemodialysis patients. Nephrology Dialysis Transplantation 2003;18(11):2359-63. [MEDLINE: ] - PubMed
Schunemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
SONG 2017
-
- SONG Initiative. The SONG Handbook Version 1.0. www.songinitiative.org/reports-and-publications/ (accessed 13 August 2018).
Staessen 1981
-
- Staessen J, Lijnen P, Fagard R, Verschueren LJ, Amery A. Rise in plasma concentration of aldosterone during long-term angiotensin II suppression. Journal of Endocrinology 1981;91(3):457-65. [MEDLINE: ] - PubMed
Steigerwalt 2007
-
- Steigerwalt S, Zafar A, Mesiha N, Gardin J, Provenzano R. Role of aldosterone in left ventricular hypertrophy among African-American patients with end-stage renal disease on hemodialysis. American Journal of Nephrology 2007;27(2):159-63. [MEDLINE: ] - PubMed
USRDS 2017a
-
- US Renal Data System. 2017 Annual Data Report. Chapter 8: Cardiovascular Disease in Patients with ESRD. www.usrds.org/media/1687/v2_c08_cvd_17.pdf (accessed 14 December 2020).
USRDS 2017b
-
- US Renal Data System. 2017 Annual Data Report. Chapter 5: Mortality. www.usrds.org/media/1681/v2_c05_mortality_17.pdf (accessed 14 December 2020).
Yancy 2013
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2013;62(16):e147-239. [MEDLINE: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

