Mechanical loading of bioengineered skeletal muscle in vitro recapitulates gene expression signatures of resistance exercise in vivo
- PMID: 33586196
- PMCID: PMC8653897
- DOI: 10.1002/jcp.30328
Mechanical loading of bioengineered skeletal muscle in vitro recapitulates gene expression signatures of resistance exercise in vivo
Abstract
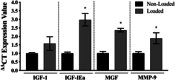
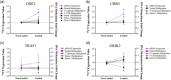
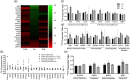
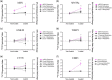
Understanding the role of mechanical loading and exercise in skeletal muscle (SkM) is paramount for delineating the molecular mechanisms that govern changes in muscle mass. However, it is unknown whether loading of bioengineered SkM in vitro adequately recapitulates the molecular responses observed after resistance exercise (RE) in vivo. To address this, the transcriptional and epigenetic (DNA methylation) responses were compared after mechanical loading in bioengineered SkM in vitro and after RE in vivo. Specifically, genes known to be upregulated/hypomethylated after RE in humans were analyzed. Ninety-three percent of these genes demonstrated similar changes in gene expression post-loading in the bioengineered muscle when compared to acute RE in humans. Furthermore, similar differences in gene expression were observed between loaded bioengineered SkM and after programmed RT in rat SkM tissue. Hypomethylation occurred for only one of the genes analysed (GRIK2) post-loading in bioengineered SkM. To further validate these findings, DNA methylation and mRNA expression of known hypomethylated and upregulated genes post-acute RE in humans were also analyzed at 0.5, 3, and 24 h post-loading in bioengineered muscle. The largest changes in gene expression occurred at 3 h, whereby 82% and 91% of genes responded similarly when compared to human and rodent SkM respectively. DNA methylation of only a small proportion of genes analyzed (TRAF1, MSN, and CTTN) significantly increased post-loading in bioengineered SkM alone. Overall, mechanical loading of bioengineered SkM in vitro recapitulates the gene expression profile of human and rodent SkM after RE in vivo. Although some genes demonstrated differential DNA methylation post-loading in bioengineered SkM, such changes across the majority of genes analyzed did not closely mimic the epigenetic response to acute-RE in humans.
Keywords: DNA methylation; bioengineering; fibrin; gene expression; mechanical loading; skeletal muscle.
© 2021 The Authors. Journal of Cellular Physiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures



References
-
- Aguilar‐Agon, K. W. , Capel, A. J. , Fleming, J. W. , Player, D. J. , Martin, N. R. W. , & Lewis, M. P. (2020). Mechanical loading of tissue engineered skeletal muscle prevents dexamethasone induced myotube atrophy. Journal of Muscle Research and Cell Motility. 10.1007/s10974-020-09589-0 - DOI - PMC - PubMed
-
- Baar, K. , & Esser, K. (1999). Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. American Journal of Physiology: Cell Physiology, 276, C120–C127. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

