Endothelium-derived semaphorin 3G attenuates ischemic retinopathy by coordinating β-catenin-dependent vascular remodeling
- PMID: 33586674
- PMCID: PMC7880421
- DOI: 10.1172/JCI135296
Endothelium-derived semaphorin 3G attenuates ischemic retinopathy by coordinating β-catenin-dependent vascular remodeling
Abstract
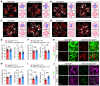
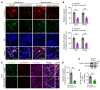
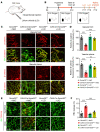
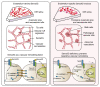
Abnormal angiogenesis and regression of the diseased retinal vasculature are key processes associated with ischemic retinopathies, but the underlying mechanisms that regulate vascular remodeling remain poorly understood. Here, we confirmed the specific expression of semaphorin 3G (Sema3G) in retinal endothelial cells (ECs), which was required for vascular remodeling and the amelioration of ischemic retinopathy. We found that Sema3G was elevated in the vitreous fluid of patients with proliferative diabetic retinopathy (PDR) and in the neovascularization regression phase of oxygen-induced retinopathy (OIR). Endothelial-specific Sema3G knockout mice exhibited decreased vessel density and excessive matrix deposition in the retinal vasculature. Moreover, loss of Sema3G aggravated pathological angiogenesis in mice with OIR. Mechanistically, we demonstrated that HIF-2α directly regulated Sema3G transcription in ECs under hypoxia. Sema3G coordinated the functional interaction between β-catenin and VE-cadherin by increasing β-catenin stability in the endothelium through the neuropilin-2 (Nrp2)/PlexinD1 receptor. Furthermore, Sema3G supplementation enhanced healthy vascular network formation and promoted diseased vasculature regression during blood vessel remodeling. Overall, we deciphered the endothelium-derived Sema3G-dependent events involved in modulating physiological vascular remodeling and regression of pathological blood vessels for reparative vascular regeneration. Our findings shed light on the protective effect of Sema3G in ischemic retinopathies.
Trial registration: ClinicalTrials.gov NCT03506750.
Keywords: Endothelial cells; Retinopathy; Vascular Biology.
Conflict of interest statement
Figures










References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

