Use of amnion-derived cellular cytokine solution for the treatment of gingivitis: A 2-week safety, dose-ranging, proof-of-principle randomized trial
- PMID: 33586783
- PMCID: PMC8518950
- DOI: 10.1002/JPER.20-0800
Use of amnion-derived cellular cytokine solution for the treatment of gingivitis: A 2-week safety, dose-ranging, proof-of-principle randomized trial
Abstract
Background: A 6-week Phase I clinical trial was performed to primarily evaluate the safety and secondarily determine the preliminary efficacy of a novel biological solution, ST266, comprised of a mixture of cytokines, growth factors, nucleic acids, and lipids secreted by cultured amnion-derived multipotent progenitor cells on gingival inflammation.
Methods: Fifty-four adults with gingivitis/periodontitis were randomly assigned to 1X ST266 or diluted 0.3X ST266 or saline topically applied on facial/lingual gingiva (20 µL/tooth). Safety was assessed through oral soft/hard tissue exam, adverse events, and routine laboratory tests. Efficacy was assessed by modified gingival index (MGI), bleeding on probing, plaque index, probing depth (PD), and clinical attachment level (CAL). Assessments were performed on day 0, 8, 12, and 42. ST266 and saline applied daily starting at day 0 through day 12 except weekend days. Plasma was analyzed for safety and proinflammatory cytokines, interleukin (IL)-1β, IL-6, tumor necrosis factor-alpha, and interferon gamma. Gingival crevicular fluid (GCF) was analyzed for the same cytokines. Subgingival plaque was primarily analyzed by checkerboard DNA-DNA hybridization. Comparisons with saline were modeled through a generalized estimating equations method adjusting for baseline.
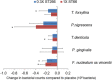
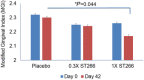
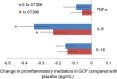
Results: No safety concern was found related to ST266. Statistically significant reduction in MGI was noted at day 42 by 1X ST266 compared with saline (P = 0.044). PD and CAL were reduced by both doses of ST266 at day 42 (P <0.01) and by 1X ST266 at day 12 (P <0.05). GCF IL-1β and IL-6 levels were reduced by both doses of ST266 at day 12 (P <0.05, P <0.01, respectively). IL-6 was also significantly reduced in plasma of both ST266 groups (P <0.05). Significant reductions in red complex bacteria were detected in both ST266 doses.
Conclusions: In this "first in human oral cavity" study, topical ST266 was safe and effective in reducing gingival inflammation in 6 weeks. Longitudinal studies with large sample sizes are warranted to assess the therapeutic value of this novel host modulatory compound in the treatment of periodontal diseases.
Keywords: cytokines; gingival crevicular fluid; gingivitis; host modulation; inflammation; periodontal disease.
© 2021 The Authors. Journal of Periodontology published by Wiley Periodicals LLC on behalf of American Academy of Periodontology.
Conflict of interest statement
All authors except Drs. Steed and Van Dyke report no conflicts of interest related to this study. Dr. Steed is the Executive VP of Medical Affairs at Noveome Biotherapeutics, owns stocks, and filed multiple patents on ST266. Dr. Van Dyke has been a paid advisor and owns stocks in Noveome Biotherapeutics.
Figures
References
-
- Heitz‐Mayfield LJ, Lang NP. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontol 2000. 2013;62:218‐231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

