Damage-Associated Molecular Patterns As Double-Edged Swords in Sepsis
- PMID: 33587003
- PMCID: PMC8817718
- DOI: 10.1089/ars.2021.0008
Damage-Associated Molecular Patterns As Double-Edged Swords in Sepsis
Abstract
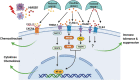
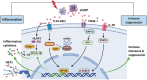
Significance: Sepsis is defined as a life-threatening organ dysfunction caused by dysregulated host response to infection. This leads to an uncontrolled inflammatory response at the onset of infection, followed by immunosuppression. The development of a specific treatment modality for sepsis is still challenging, reflecting our inadequate understanding of its pathophysiology. Understanding the mechanism and transition of the early hyperinflammation to late stage of immunosuppression in sepsis is critical for developing sepsis therapeutics. Recent Advances: Damage-associated molecular patterns (DAMPs) are intracellular molecules and released upon tissue injury and cell death in sepsis. DAMPs are recognized by pattern recognition receptors to initiate inflammatory cascades. DAMPs not only elicit an inflammatory response but also they subsequently induce immunosuppression, both are equally important for exacerbating sepsis. Recent advances on a new DAMP, extracellular cold-inducible RNA-binding protein for fueling inflammation and immunosuppression in sepsis, have added a new avenue into the dual functions of DAMPs in sepsis. Critical Issues: The molecular modification of DAMPs and their binding to pattern recognition receptors transit dynamically by the cellular environment in pathophysiologic conditions. Correlation between the dynamic changes of the impacts of DAMPs and the clinical outcomes in sepsis still lacks adequate understanding. Here, we focus on the impacts of DAMPs that cause inflammation as well as induce immunosuppression in sepsis. We further discuss the therapeutic potential by targeting DAMPs to attenuate inflammation and immunosuppression for mitigating sepsis. Future Directions: Uncovering pathways of the transition from inflammation to immunosuppression of DAMPs is a potential therapeutic avenue for mitigating sepsis.
Keywords: ATP; DAMP; HMGB1; eCIRP; histones; immunosuppression; inflammation.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hagele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu M, Schwarzenberger C, Hohenstein B, Hugo C, Uhl B, Reichel CA, Krombach F, Monestier M, Liapis H, Moreth K, Schaefer L, and Anders HJ. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol 23: 1375–1388, 2012. - PMC - PubMed
-
- Andersson U, Yang H, and Harris H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin Immunol 38: 40–48, 2018. - PubMed
-
- Barnay-Verdier S, Fattoum L, Borde C, Kaveri S, Gibot S, and Marechal V. Emergence of autoantibodies to HMGB1 is associated with survival in patients with septic shock. Intensive Care Med 37: 957–962, 2011. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

