α-Synuclein plasma membrane localization correlates with cellular phosphatidylinositol polyphosphate levels
- PMID: 33587036
- PMCID: PMC7929559
- DOI: 10.7554/eLife.61951
α-Synuclein plasma membrane localization correlates with cellular phosphatidylinositol polyphosphate levels
Abstract
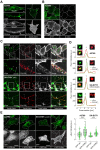
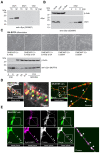
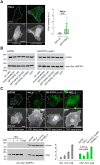
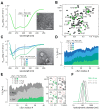
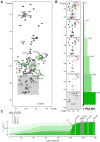
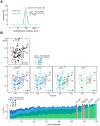
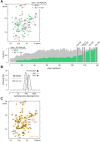
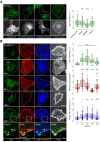
The Parkinson's disease protein α-synuclein (αSyn) promotes membrane fusion and fission by interacting with various negatively charged phospholipids. Despite postulated roles in endocytosis and exocytosis, plasma membrane (PM) interactions of αSyn are poorly understood. Here, we show that phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3), two highly acidic components of inner PM leaflets, mediate PM localization of endogenous pools of αSyn in A2780, HeLa, SK-MEL-2, and differentiated and undifferentiated neuronal SH-SY5Y cells. We demonstrate that αSyn binds to reconstituted PIP2 membranes in a helical conformation in vitro and that PIP2 synthesizing kinases and hydrolyzing phosphatases reversibly redistribute αSyn in cells. We further delineate that αSyn-PM targeting follows phosphoinositide-3 kinase (PI3K)-dependent changes of cellular PIP2 and PIP3 levels, which collectively suggests that phosphatidylinositol polyphosphates contribute to αSyn's function(s) at the plasma membrane.
Keywords: A2780; HeLa; SH-SY5Y; SK-MEL-2; human cell lines; neuroscience.
© 2021, Jacob et al.
Conflict of interest statement
RJ, CE, AD, DM, PS No competing interests declared
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

