Investigation into the difference in mitochondrial-cytosolic calcium coupling between adult cardiomyocyte and hiPSC-CM using a novel multifunctional genetic probe
- PMID: 33587181
- PMCID: PMC8100988
- DOI: 10.1007/s00424-021-02524-3
Investigation into the difference in mitochondrial-cytosolic calcium coupling between adult cardiomyocyte and hiPSC-CM using a novel multifunctional genetic probe
Abstract
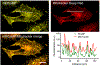
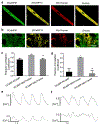
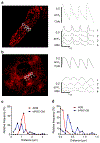
Ca2+ cycling plays a critical role in regulating cardiomyocyte (CM) function under both physiological and pathological conditions. Mitochondria have been implicated in Ca2+ handling in adult cardiomyocytes (ACMs). However, little is known about their role in the regulation of Ca2+ dynamics in human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). In the present study, we developed a multifunctional genetically encoded Ca2+ probe capable of simultaneously measuring cytosolic and mitochondrial Ca2+ in real time. Using this novel probe, we determined and compared mitochondrial Ca2+ activity and the coupling with cytosolic Ca2+ dynamics in hiPSC-CMs and ACMs. Our data showed that while ACMs displayed a highly coordinated beat-by-beat response in mitochondrial Ca2+ in sync with cytosolic Ca2+, hiPSC-CMs showed high cell-wide variability in mitochondrial Ca2+ activity that is poorly coordinated with cytosolic Ca2+. We then revealed that mitochondrial-sarcoplasmic reticulum (SR) tethering, as well as the inter-mitochondrial network connection, is underdeveloped in hiPSC-CM compared to ACM, which may underlie the observed spatiotemporal decoupling between cytosolic and mitochondrial Ca2+ dynamics. Finally, we showed that knockdown of mitofusin-2 (Mfn2), a protein tethering mitochondria and SR, led to reduced cytosolic-mitochondrial Ca2+ coupling in ACMs, albeit to a lesser degree compared to hiPSC-CMs, suggesting that Mfn2 is a potential engineering target for improving mitochondrial-cytosolic Ca2+ coupling in hiPSC-CMs. Physiological relevance: The present study will advance our understanding of the role of mitochondria in Ca2+ handling and cycling in CMs, and guide the development of hiPSC-CMs for healing injured hearts.
Keywords: Ca2+ cycling; Genetically encoded Ca2+ probe; Mitochondrial network; hiPSC-CM.
Conflict of interest statement
Conflict of interest
None.
Figures



Similar articles
-
Cellular calcium handling and electrophysiology are modulated by chronic physiological pacing in human induced pluripotent stem cell-derived cardiomyocytes.Am J Physiol Heart Circ Physiol. 2024 Nov 1;327(5):H1244-H1254. doi: 10.1152/ajpheart.00536.2024. Epub 2024 Sep 20. Am J Physiol Heart Circ Physiol. 2024. PMID: 39302711
-
Comparable calcium handling of human iPSC-derived cardiomyocytes generated by multiple laboratories.J Mol Cell Cardiol. 2015 Aug;85:79-88. doi: 10.1016/j.yjmcc.2015.05.003. Epub 2015 May 14. J Mol Cell Cardiol. 2015. PMID: 25982839 Free PMC article.
-
Integrins Increase Sarcoplasmic Reticulum Activity for Excitation-Contraction Coupling in Human Stem Cell-Derived Cardiomyocytes.Int J Mol Sci. 2022 Sep 19;23(18):10940. doi: 10.3390/ijms231810940. Int J Mol Sci. 2022. PMID: 36142853 Free PMC article.
-
SR-mitochondria communication in adult cardiomyocytes: A close relationship where the Ca2+ has a lot to say.Arch Biochem Biophys. 2019 Mar 15;663:259-268. doi: 10.1016/j.abb.2019.01.026. Epub 2019 Jan 24. Arch Biochem Biophys. 2019. PMID: 30685253 Free PMC article. Review.
-
Ca2+ signaling of human pluripotent stem cells-derived cardiomyocytes as compared to adult mammalian cardiomyocytes.Cell Calcium. 2020 Sep;90:102244. doi: 10.1016/j.ceca.2020.102244. Epub 2020 Jun 13. Cell Calcium. 2020. PMID: 32585508 Free PMC article. Review.
Cited by
-
Mitochondrial dysfunction is a key link involved in the pathogenesis of sick sinus syndrome: a review.Front Cardiovasc Med. 2024 Oct 29;11:1488207. doi: 10.3389/fcvm.2024.1488207. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39534498 Free PMC article. Review.
-
Characterization of the far-red fluorescent probe MitoView 633 for dynamic mitochondrial membrane potential measurement.Front Physiol. 2023 Oct 23;14:1257739. doi: 10.3389/fphys.2023.1257739. eCollection 2023. Front Physiol. 2023. PMID: 37936577 Free PMC article.
-
Cardiac calcium regulation in human induced pluripotent stem cell cardiomyocytes: Implications for disease modeling and maturation.Front Cell Dev Biol. 2023 Jan 18;10:986107. doi: 10.3389/fcell.2022.986107. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36742199 Free PMC article. Review.
References
-
- Akerboom J, Rivera JDV, Guilbe MMR, Malave ECA, Hernandez HH, Tian L, Hires SA, Marvin JS, Looger LL, Schreiter ER (2009) Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational designs. J Biol Chem 284:6455–6464. doi:10.1074/jbc.M807657200 - DOI - PMC - PubMed
-
- Ben-Ari M, Naor S, Zeevi-Levin N, Schick R, Jehuda RB, Reiter I, Raveh A, Grijnevitch I, Barak O, Rosen MR, Weissman A, Binah O (2016) Developmental changes in electrophysiological characteristics of human-induced pluripotent stem cell-derived cardiomyocytes. Heart Rhythm 13:2379–2387. doi:10.1016/j.hrthm.2016.08.045 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

