Ex Vivo Expanded Donor Alloreactive Regulatory T Cells Lose Immunoregulatory, Proliferation, and Antiapoptotic Markers After Infusion Into ATG-lymphodepleted, Nonhuman Primate Heart Allograft Recipients
- PMID: 33587433
- PMCID: PMC8239063
- DOI: 10.1097/TP.0000000000003617
Ex Vivo Expanded Donor Alloreactive Regulatory T Cells Lose Immunoregulatory, Proliferation, and Antiapoptotic Markers After Infusion Into ATG-lymphodepleted, Nonhuman Primate Heart Allograft Recipients
Abstract
Background: Regulatory T cell (Treg) therapy is a promising approach to amelioration of allograft rejection and promotion of organ transplant tolerance. However, the fate of infused Treg, and how this relates to their therapeutic efficacy using different immunosuppressive regimens is poorly understood. Our aim was to analyze the tissue distribution, persistence, replicative activity and phenotypic stability of autologous, donor antigen alloreactive Treg (darTreg) in anti-thymocyte globulin (ATG)-lymphodepleted, heart-allografted cynomolgus monkeys.
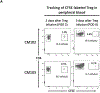
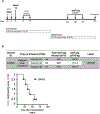
Methods: darTreg were expanded ex vivo from flow-sorted, circulating Treg using activated donor B cells and infused posttransplant into recipients of major histocompatibility complex-mismatched heart allografts. Fluorochrome-labeled darTreg were identified and characterized in peripheral blood, lymphoid, and nonlymphoid tissues and the graft by flow cytometric analysis.
Results: darTreg selectively suppressed autologous T cell responses to donor antigens in vitro. However, following their adoptive transfer after transplantation, graft survival was not prolonged. Early (within 2 wk posttransplant; under ATG, tacrolimus, and anti-IL-6R) or delayed (6-8 wk posttransplant; under rapamycin) darTreg infusion resulted in a rapid decline in transferred darTreg in peripheral blood. Following their early or delayed infusion, labeled cells were evident in lymphoid and nonlymphoid organs and the graft at low percentages (<4% CD4+ T cells). Notably, infused darTreg showed reduced expression of immunoregulatory molecules (Foxp3 and CTLA4), Helios, the proliferative marker Ki67 and antiapoptotic Bcl2, compared with preinfusion darTreg and endogenous CD4+CD25hi Treg.
Conclusions: Lack of therapeutic efficacy of infused darTreg in lymphodepleted heart graft recipients appears to reflect loss of a regulatory signature and proliferative and survival capacity shortly after infusion.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures








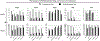




References
-
- Sicard A, Boardman DA, Levings MK. Taking regulatory T-cell therapy one step further. Curr Opin Organ Transplant 2018;23:509–515. - PubMed
-
- Romano M, Tung SL, Smyth LA, et al. Treg therapy in transplantation: a general overview. Transpl Int 2017;30:745–753. - PubMed
-
- Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol 2012;12:417–430. - PubMed
-
- Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant 2007;7:1457–1463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

