Engineering of Biocompatible Coacervate-Based Synthetic Cells
- PMID: 33587612
- PMCID: PMC7908014
- DOI: 10.1021/acsami.0c19052
Engineering of Biocompatible Coacervate-Based Synthetic Cells
Abstract
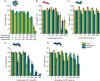
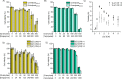
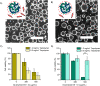
Polymer-stabilized complex coacervate microdroplets have emerged as a robust platform for synthetic cell research. Their unique core-shell properties enable the sequestration of high concentrations of biologically relevant macromolecules and their subsequent release through the semipermeable membrane. These unique properties render the synthetic cell platform highly suitable for a range of biomedical applications, as long as its biocompatibility upon interaction with biological cells is ensured. The purpose of this study is to investigate how the structure and formulation of these coacervate-based synthetic cells impact the viability of several different cell lines. Through careful examination of the individual synthetic cell components, it became evident that the presence of free polycation and membrane-forming polymer had to be prevented to ensure cell viability. After closely examining the structure-toxicity relationship, a set of conditions could be found whereby no detrimental effects were observed, when the artificial cells were cocultured with RAW264.7 cells. This opens up a range of possibilities to use this modular system for biomedical applications and creates design rules for the next generation of coacervate-based, biomedically relevant particles.
Keywords: biocompatibility; block copolymers; complex coacervates; polycations; protocells; self-assembly; synthetic cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

