Orexin Modulation of VTA Dopamine Neuron Activity: Relevance to Schizophrenia
- PMID: 33587746
- PMCID: PMC8059491
- DOI: 10.1093/ijnp/pyaa080
Orexin Modulation of VTA Dopamine Neuron Activity: Relevance to Schizophrenia
Abstract
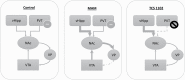
Background: The hippocampus is a region consistently implicated in schizophrenia and has been advanced as a therapeutic target for positive, negative, and cognitive deficits associated with the disease. Recently, we reported that the paraventricular nucleus of the thalamus (PVT) works in concert with the ventral hippocampus to regulate dopamine system function; however, the PVT has yet to be investigated as target for the treatment of the disease. Given the dense expression of orexin receptors in the thalamus, we believe these to be a possible target for pharmacological regulation of PVT activity.
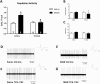
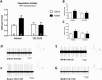
Methods: Here we used the methylazoxymethanol acetate (MAM) rodent model, which displays pathological alterations consistent with schizophrenia to determine whether orexin receptor blockade can restore ventral tegmental area dopamine system function. We measured dopamine neuron population activity, using in vivo electrophysiology, following administration of the dual orexin antagonist, TCS 1102 (both intraperitoneal and intracranial into the PVT in MAM- and saline-treated rats), and orexin A and B peptides (intracranial into the PVT in naïve rats).
Results: Aberrant dopamine system function in MAM-treated rats was normalized by the systemic administration of TCS 1102. To investigate the potential site of action, the orexin peptides A and B were administered directly into the PVT, where they significantly increased ventral tegmental area dopamine neuron population activity in control rats. In addition, the direct administration of TCS 1102 into the PVT reproduced the beneficial effects seen with the systemic administration in MAM-treated rats.
Conclusion: Taken together, these data suggest the orexin system may represent a novel site of therapeutic intervention for psychosis via an action in the PVT.
Keywords: Schizophrenia; dopamine; orexins; paraventricular nucleus of the thalamus.
© The Author(s) 2021. Published by Oxford University Press on behalf of CINP.
Figures

Similar articles
-
Orexin receptor antagonists reverse aberrant dopamine neuron activity and related behaviors in a rodent model of stress-induced psychosis.Transl Psychiatry. 2021 Feb 8;11(1):114. doi: 10.1038/s41398-021-01235-8. Transl Psychiatry. 2021. PMID: 33558469 Free PMC article.
-
Convergent Inputs from the Hippocampus and Thalamus to the Nucleus Accumbens Regulate Dopamine Neuron Activity.J Neurosci. 2018 Dec 12;38(50):10607-10618. doi: 10.1523/JNEUROSCI.2629-16.2018. Epub 2018 Oct 24. J Neurosci. 2018. PMID: 30355626 Free PMC article.
-
A novel α5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia.Neuropsychopharmacology. 2011 Aug;36(9):1903-11. doi: 10.1038/npp.2011.76. Epub 2011 May 11. Neuropsychopharmacology. 2011. PMID: 21562483 Free PMC article.
-
Insights on current and novel antipsychotic mechanisms from the MAM model of schizophrenia.Neuropharmacology. 2020 Feb;163:107632. doi: 10.1016/j.neuropharm.2019.05.009. Epub 2019 May 8. Neuropharmacology. 2020. PMID: 31077730 Free PMC article. Review.
-
Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia.Neurotox Res. 2008 Oct;14(2-3):97-104. doi: 10.1007/BF03033801. Neurotox Res. 2008. PMID: 19073417 Free PMC article. Review.
Cited by
-
Dual orexin/hypocretin receptor antagonism attenuates NMDA receptor hypofunction-induced attentional impairments in a rat model of schizophrenia.Behav Brain Res. 2023 Jul 26;450:114497. doi: 10.1016/j.bbr.2023.114497. Epub 2023 May 16. Behav Brain Res. 2023. PMID: 37196827 Free PMC article.
-
Understanding translational research in schizophrenia: A novel insight into animal models.Mol Biol Rep. 2023 Apr;50(4):3767-3785. doi: 10.1007/s11033-023-08241-7. Epub 2023 Jan 24. Mol Biol Rep. 2023. PMID: 36692676 Free PMC article. Review.
-
Gestational buprenorphine exposure disrupts dopamine neuron activity and related behaviors in adulthood.eNeuro. 2022 Jul 18;9(4):ENEURO.0499-21.2022. doi: 10.1523/ENEURO.0499-21.2022. Online ahead of print. eNeuro. 2022. PMID: 35851301 Free PMC article.
-
Inhibition of hippocampal or thalamic inputs to the nucleus accumbens reverses stress-induced alterations in dopamine system function.Int J Neuropsychopharmacol. 2025 Jun 6;28(6):pyaf034. doi: 10.1093/ijnp/pyaf034. Int J Neuropsychopharmacol. 2025. PMID: 40408392 Free PMC article.
-
Neurobiology of the Orexin System and Its Potential Role in the Regulation of Hedonic Tone.Brain Sci. 2022 Jan 24;12(2):150. doi: 10.3390/brainsci12020150. Brain Sci. 2022. PMID: 35203914 Free PMC article. Review.
References
-
- Abi-Dargham A (2004) Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol 7 Suppl 1:S1–S5. - PubMed
-
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M (1998) Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 155:761–767. - PubMed
-
- Andreasen NC (1997) The role of the thalamus in schizophrenia. Can J Psychiatry 42:27–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

