PRRX1 deficiency induces mesenchymal-epithelial transition through PITX2/miR-200-dependent SLUG/CTNNB1 regulation in hepatocellular carcinoma
- PMID: 33587761
- PMCID: PMC8177778
- DOI: 10.1111/cas.14853
PRRX1 deficiency induces mesenchymal-epithelial transition through PITX2/miR-200-dependent SLUG/CTNNB1 regulation in hepatocellular carcinoma
Abstract
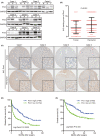
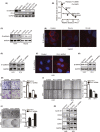
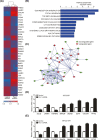
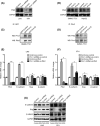
Metastasis is a major obstacle to better prognosis in patients with hepatocellular carcinoma (HCC). Mesenchymal-epithelial transition (MET) is the driving force for metastatic colonization in which E-cadherin re-expression is a critical procedure. It has been reported that the loss of paired-related homeobox transcription factor 1 (PRRX1) is required for cancer cell metastasis. However, the role of PRRX1 in MET and how its downregulation triggers E-cadherin re-expression are unknown. In this study, we performed a systematic, mechanistic study regarding the role of PRRX1 in MET of HCC. We observed PRRX1 downregulation in HCC tissues, which correlated with early metastasis and short overall survival. Overexpression of PRRX1 induced epithelial-mesenchymal transition (EMT), but did not promote metastasis formation, while knockdown of PRRX1 promoted metastasis and colonization of circulating HCC cells as shown in animal model. PRRX1 protein levels reversely correlated with E-cadherin levels in HCC cell lines. PRRX1 knockdown promoted E-cadherin re-expression and cell proliferation and inhibited cell invasion and migration. The microarray results showed that PRRX1 deficiency regulated extracellular matrix (ECM) interaction, focal adhesion, TGF-β signaling and cancer pathways. PRRX1 knockdown upregulated paired-like homeodomain 2 (PITX2) and inhibited catenin beta 1 (CTNNB1) and SNAIL family zinc finger 2 (SLUG). Silencing of PITX2 reversed CTNNB1 and SLUG inhibition and E-cadherin re-expression. PITX2 upregulation increased miR-200a and miR-200b/429, which further inhibited the transcription of CTNNB1 and SLUG, respectively, thus abrogating the inhibitory effect on E-cadherin. In conclusion, our data showed that the downregulation of PRRX1 induced E-cadherin re-expression through PITX2/miR-200a/CTNNB1 and PITX2/miR-200b/429/SLUG pathway.
Keywords: E-cadherin re-expression; PITX2; PRRX1; hepatocellular carcinoma; mesenchymal-epithelial transition.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflict of interest.
Figures


Similar articles
-
MiR-122 inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting Snail1 and Snail2 and suppressing WNT/β-cadherin signaling pathway.Exp Cell Res. 2017 Nov 15;360(2):210-217. doi: 10.1016/j.yexcr.2017.09.010. Epub 2017 Sep 8. Exp Cell Res. 2017. PMID: 28890291
-
MicroRNA-148a suppresses the epithelial-mesenchymal transition and metastasis of hepatoma cells by targeting Met/Snail signaling.Oncogene. 2014 Jul 31;33(31):4069-76. doi: 10.1038/onc.2013.369. Epub 2013 Sep 9. Oncogene. 2014. PMID: 24013226
-
PHF8 upregulation contributes to autophagic degradation of E-cadherin, epithelial-mesenchymal transition and metastasis in hepatocellular carcinoma.J Exp Clin Cancer Res. 2018 Sep 4;37(1):215. doi: 10.1186/s13046-018-0890-4. J Exp Clin Cancer Res. 2018. PMID: 30180906 Free PMC article.
-
The Role of the MiR-181 Family in Hepatocellular Carcinoma.Cells. 2024 Jul 31;13(15):1289. doi: 10.3390/cells13151289. Cells. 2024. PMID: 39120319 Free PMC article. Review.
-
Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer.Tumour Biol. 2014 Aug;35(8):7335-42. doi: 10.1007/s13277-014-2163-y. Epub 2014 Jun 2. Tumour Biol. 2014. PMID: 24880591 Review.
Cited by
-
Role of Epithelial-to-Mesenchymal Transition for the Generation of Circulating Tumors Cells and Cancer Cell Dissemination.Cancers (Basel). 2022 Nov 8;14(22):5483. doi: 10.3390/cancers14225483. Cancers (Basel). 2022. PMID: 36428576 Free PMC article. Review.
-
Text-Mining Approach to Identify Hub Genes of Cancer Metastasis and Potential Drug Repurposing to Target Them.J Clin Med. 2022 Apr 11;11(8):2130. doi: 10.3390/jcm11082130. J Clin Med. 2022. PMID: 35456223 Free PMC article.
-
What is the Impact of Endothelial-to-Mesenchymal Transition in Solid Tumours: A Qualitative Systematic Review and Quantitative Meta-Analysis.Int J Biol Sci. 2025 Feb 24;21(5):2155-2178. doi: 10.7150/ijbs.107045. eCollection 2025. Int J Biol Sci. 2025. PMID: 40083695 Free PMC article.
-
PRRX1/FOXM1 reduces gemcitabin-induced cytotoxicity by regulating autophagy in bladder cancer.Transl Androl Urol. 2022 Aug;11(8):1116-1129. doi: 10.21037/tau-22-415. Transl Androl Urol. 2022. PMID: 36092834 Free PMC article.
-
The Regulatory Role of PRRX1 in Cancer Epithelial-Mesenchymal Transition.Onco Targets Ther. 2021 Jul 16;14:4223-4229. doi: 10.2147/OTT.S316102. eCollection 2021. Onco Targets Ther. 2021. PMID: 34295164 Free PMC article. Review.
References
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559‐1564. - PubMed
-
- Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65:798‐808. - PubMed
-
- Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128‐134. - PubMed
-
- Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996‐6001; discussion 6000‐5991. - PubMed
-
- Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21‐45. - PubMed
MeSH terms
Substances
Grants and funding
- 81572393/National Natural Science Foundation of China
- 81602093: 81602054/National Natural Science Foundation of China
- BK20160118/Natural Science Foundation of Jiangsu Province
- BK20151042/Natural Science Foundation of Jiangsu Province
- ZKX15020/Key Project supported by the Medical Science and Technology Development Foundation, Nanjing Municipality Health Bureau
- ZKX17022/Key Project supported by the Medical Science and Technology Development Foundation, Nanjing Municipality Health Bureau
- 021414380215/Fundamental Research Funds for the Central Universities
- 021414380242/Fundamental Research Funds for the Central Universities
- 021414380258/Fundamental Research Funds for the Central Universities
- Applied Basic Research of Changzhou Technology Bureau
- ZD201906/Major Science and Technology Project of Changzhou Health Committee
- Chen Xiao-Ping Foundation for the Development of Science and Technology of Hubei Province: CXPJJH12000001-2020318
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

